Ghana National Malaria Elimination Programme (NMEP) in line with the WHO aims to minimize malaria morbidity. It aims to approve access to vector control, diagnosis, and treatment, as well as strengthen surveillance and reporting [13]. This study…
Blog
-
Just a moment…
Just a moment… This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Continue Reading
-
Google Gets Manipulative with New Hide Ads Feature – TechRepublic
- Google Gets Manipulative with New Hide Ads Feature TechRepublic
- We’re improving navigation and introducing a new control for ads on Google Search. The Keyword
- Google revamps search ads — See what’s paid, hide what you don’t want
Continue Reading
-

Facilitators and barriers of malaria prevention and treatment services to pregnant women in Ethiopia: a multi-level health system analysis, 2025 | Malaria Journal
WHO. World malaria report. Geneva: World Health Organization; 2024.
Bauserman M, Conroy AL, North K, Patterson J, Bose C, Meshnick S. An overview of malaria in pregnancy. Semin Perinatol….
Continue Reading
-
JPMorgan’s Profit Jumps as Business Booms on Main Street, Wall Street – The Wall Street Journal
- JPMorgan’s Profit Jumps as Business Booms on Main Street, Wall Street The Wall Street Journal
- JPMorgan Chase tops estimates as trading revenue hits a record of nearly $9 billion CNBC
- Dimon Calls Out Weaker Job Market, Inflation as Provisions Rise Bloomberg.com
- US banks: JPM and Wells Fargo rise after Q3 results TradingView
- JPMorgan gets Wall Street lift, warns of economy ‘softening’ American Banker
Continue Reading
-

Researchers Reveal How Plants Make Anti-tumour Drugs
UBC Okanagan doctoral student Tuan-Anh Nguyen, left, and Dr. Thu-Thuy Dang examine plant samples in their lab. Their research has uncovered how tropical trees produce mitraphylline, a rare compound with potential anti-tumour…
Continue Reading
-

Nicholas Sparks on Collaborating With M. Night Shyamalan for ‘Remain’
Nicholas Sparks and M. Night Shyamalan are more similar than you think. And though joining forces for a novel may seem like a unique choice, the pair’s paths have crossed before.
“Long time ago, when we had the original script of The…
Continue Reading
-

Fibocom Leads the FWA Evolution at NetworkX 2025 with AI-Powered Connectivity Innovations
PARIS, Oct. 14, 2025 /PRNewswire/ — Fibocom, a leading global provider of wireless communication modules and AI solutions, is showcasing its leadership in 5G Fixed Wireless…
Continue Reading
-
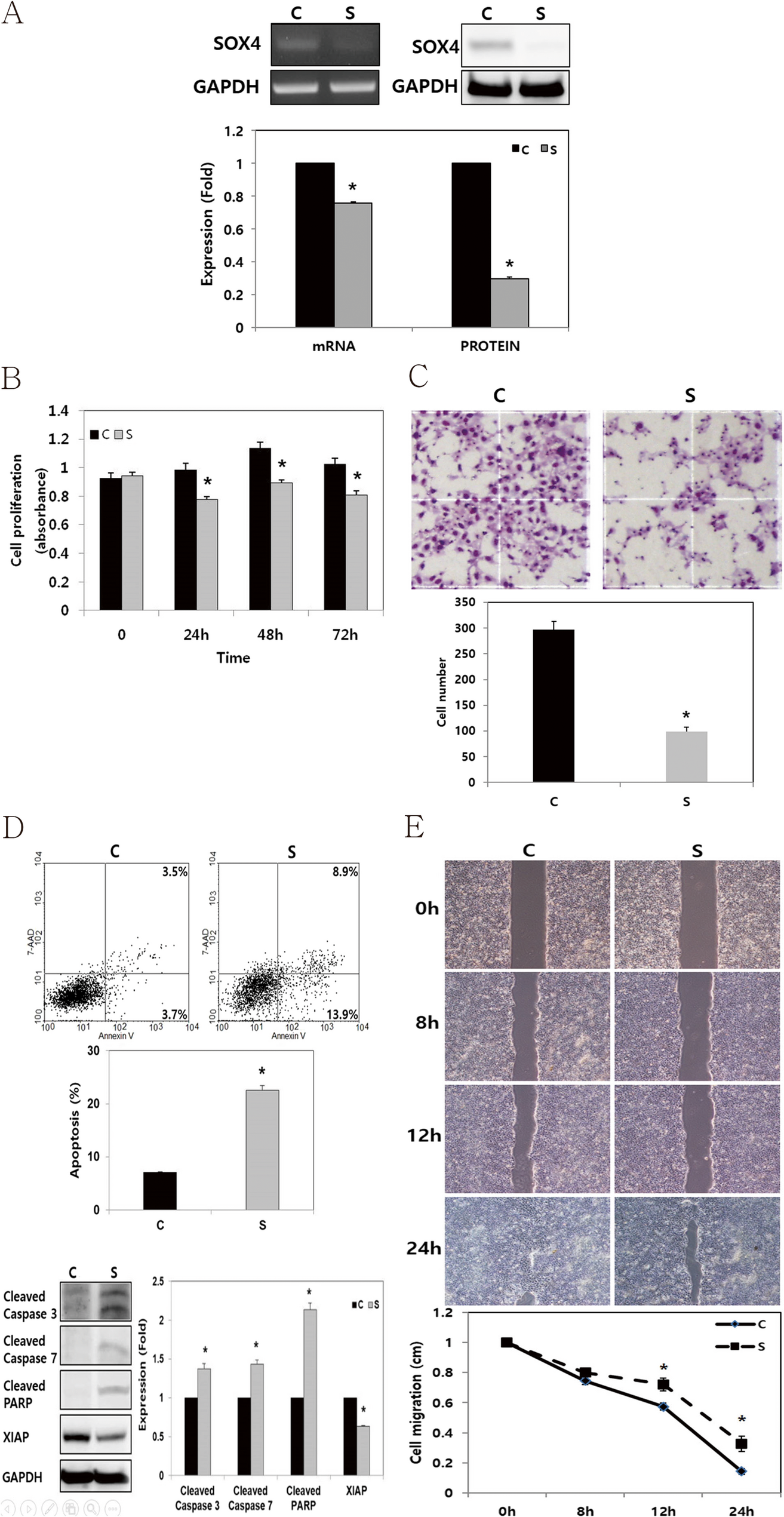
SOX4 enhances tumor progression and cisplatin resistance in orthotopic mouse xenograft model of head and neck squamous cell carcinoma | BMC Cancer
Cell culture and transfection
The HNSCC, FADU cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA). FADU cell line were cultured in DMEM/F12 medium (GIBCO®, Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS, GIBCO®, Invitrogen) and 1% penicillin/streptomycin. Cells were cultured in a Humidified incubator at 37°C with 5% CO2. To knock down endogenous SOX4 gene expression in FADU cells, small interfering RNAs (siRNAs) were utilized. FADU cells were seeded into 6-well plates at a density of 2.0 × 105 cells/well and transfected with a SOX4-specific (Bioneer, Daejeon, Korea) or a negative control siRNA (cat. no. 1027281, Qiagen, Germantown, MD, USA) using (Invitrogen) for 48 h at 37°C. The SOX4-specific si-RNA sequences were as follows: Sense, 5’- GAU AGA UGG CGC UAU CUU U-3’ and Antisense, 5’-AAA CAU AGC GCC AUC UAU C −3’. Subsequent analyses were conducted 48 h post-transfection.
RNA isolation and reverse-transcription polymerase chain reaction
Total RNA was extracted from FADU using TRIzol reagent (Invitrogen) following the manufacturer’s protocol. For reverse transcription (RT), total RNA (1 µg), M-MLV reverse transcriptase (Invitrogen), 1 µL of 2 mM dNTP mix (Enzynomics Co., Ltd., Daejeon, Korea), 2 µL of 0.1 M dithiothreitol (Invitrogen), 4 µL of 5× first strand buffer (Invitrogen), 1 µL of RNase inhibitor (Promega Corporation), and 1 µL of oligo dT (Bioneer Corporation, Daejeon, Korea) were used. Primers specific for SOX4 and Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH, Bioneer Corporation) were utilized to amplify the cDNA. Polymerase chain reaction (PCR) was performed using GoTaq DNA Polymerase and 5× Green GoTaq reaction buffer (Promega Corporation). The primer sequences employed were as follows: SOX4 forward, 5ʹ- GCA CAT GGC TGA CTA CCC C-3ʹ; SOX4 reverse, 5ʹ- GCC TTG TAC AGC GAG TGG TG-3ʹ; GAPDH forward, 5ʹ-ACC ACA GTC CAT GCC ATC AC-3ʹ; and GAPDH reverse, 5ʹ-TCC ACC CTG TTG CTG TA-3ʹ. PCR products were separated via electrophoresis on a 1% agarose gel containing ethidium bromide.
Protein isolation and western blot analysis
Cells were lysed using a radioimmunoprecipitation assay buffer (Biosesang Inc.). Protein concentrations were subsequently measured using a bicinchoninic acid assay. Protein lysates (20–30 µg per lane) were separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10%–12% gels and electrophoretically transferred onto polyvinylidene fluoride membranes. The membranes were then incubated at room temperature for 1 h with 5% bovine serum albumin (BSA; Bioshop Canada Inc.) in Tris-buffered saline (TBS) containing 0.1% Tween-20. The membranes were then washed four times for 15 min each with TBS–0.1% Tween-20. Specific proteins were identified using primary antibodies targeting GAPDH (cat. no. sc-25778; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), β-actin (cat. no. 3700 S; Cell signaling Technology, Inc.), SOX4 (cat. no. ab80261; Abcam, UK), Cleaved caspase-3 (cat. no. 9664; Cell Signaling Technology, Inc.), Cleaved caspase-7 (cat. no. 9491; Cell Signaling Technology, Inc.), X-linked inhibitor of apoptosis protein (XIAP, cat.no. sc-11426; Santa Cruze Biotechnology, Texas, USA), and Cleaved poly (ADP-ribose) polymerase (PARP; cat. no. 5625; Cell Signaling Technology, Inc.; cat. no. ab32064; Abcam). The primary antibodies were diluted at a 1:1,000 ratios in TBS–0.1% Tween-20 and incubated with the membranes for 24 h at 4 °C. Secondary antibodies, anti-rabbit (cat. no. 7074; Cell Signaling Technology, Inc.) or anti-mouse (cat. no. 7076; Cell Signaling Technology, Inc.), were diluted at 1:2,000 ratio, and incubated at room temperature for 1 h. Immunoreactive proteins were visualized using a LAS 4000 luminescence image analyzer (FUJIFILM Wako Pure Chemical Corporation) and an enhanced chemiluminescence detection system for HRP (EMD Millipore). Western blot analysis was independently conducted in triplicate.
Cell proliferation assay
Following of transfection, cells plated in 24-well plates (1 × 104 cells per well). After an additional 48 h incubation, cell viability was measured using an EZ-CyTox (WST-1) enhanced cell viability assay kit (cat. no. EZ-3000; Daeil Lab Inc.) at 37 °C for 1–2 h. Absorbance was measured at 460 nm using a microplate reader. Cell viability assays were performed in triplicate and repeated independently.
Cell invasion assay
The extent of cell invasion was evaluated by counting the number of cells that migrated through an 8.0 μm pore Transwell invasion chamber (cat. no. 3422; Costar, Inc.). The upper chamber was coated with a 1% gelatin solution and incubated for 12 h at 37 °C, followed by 12 h of drying at room temperature before the experiment. After 48 h of transfection, cells in the upper chamber were seeded at a density of 2 × 105 cells in 120 µl of 0.2% BSA (BioShop Canada, Inc.) in FBS-free DMEM. As a chemoattractant, 400 µl of 0.2% BSA in FBS-free DMEM containing fibronectin (cat. no.361635; Calbiochem, San Diego, CA, USA) was loaded into the lower chamber. After 24 h of incubation, cells that had migrated to the bottom of the Transwell membranes were stained with Diff-Quik solution (Sysmex Corporation). Using a Light microscope, the cells were then counted in five random fields at 100× magnification. Results were expressed as mean ± standard error of the number of cells per field from three independent experiments.
Cell migration assay (wound healing assay)
The cells were seeded into each well of Culture-Inserts (Ibidi GmbH) at 1.5 × 105 cells/well, following transfection. They were incubated for 24 h. Following that, each insert was detached, and the progression of cell migration was assessed by imaging at 0, 8, 12, and 24 h using an inverted microscope. Distances between gaps were normalized to 1 cm after three random sites were captured.
Apoptosis assay
Apoptosis was evaluated using an APC Annexin V assay. Following transfection, the cells were harvested via trypsinization, washed twice with phosphate-buffered saline, and resuspended in a binding buffer (cat. no. 556454; BD Biosciences, San Jose, CA, USA). After adding APC Annexin V (cat. no. 550474) and 7-amino-actinomycin D (cat. no. 559925; BD Biosciences, San Jose, CA, USA), the cells were incubated in the dark for 15 min. The samples were subsequently resuspended in 400 µl of binding buffer. They were analyzed using a FACSCalibur flow cytometer (BD Biosciences) and BD Cell Quest version 3.3 software (Becton Dickinson). Data analysis was performed using WinMDI version 2.9 (The Scripps Research Institute). Apoptosis assays were conducted independently in triplicate.
Cell irradiation or cisplatin treatment
After 48 h of transfection, the cells were maintained at 37 °C and exposed to γ-irradiation at varying doses (8–10 Gy, 137Cs, and 2.875 Gy/min) using a Gammacell 3000 Elan (Therathronics) at room temperature. A stock solution of cisplatin (10 mg/20 ml; Dong-A, Co., Ltd., Seoul, Korea) was prepared and diluted to 2.5 ~ 5 µg/ml concentrations. The cisplatin solution was incubated at 37 °C for 24 h before being used in experiments.
Establishment of SOX4 overexpressing stable cell line and an orthotopic mouse xenograft model of head and neck squamous cell carcinoma
A stable SOX4-overexpressing SCC VII mouse squamous cell carcinoma cell line was established. After transfection of pcDNA6/myc-SOX4 into SCC VII cells using Lipofectamine 2000 (Thermo Fisher Scientific, USA), transfected cells were selected by culturing in media containing blasticidin (cat. no. A11139-03; ThermoFisher, Massachusetts, USA) at 5 µg/ml concentration. The stable overexpression of SOX4 in selected clones was confirmed via Western blot analysis. Female C3H/HeJ syngeneic mice (6–8 weeks old) were purchased from OrientBio (Seongnam, South Korea), and randomly assigned to a control or SOX4 overexpression group. Furthermore, 1 × 106 of SOX4 overexpressed or controlled SCC VII cells were suspended in 70 µl of serum-free media and slowly injected into the floor of the mouth (FOM) of mice via an intraoral approach. These animal experimental procedures were approved by the Chonnam National University Animal Care and Use Committee (CNU IACUC-H-201624). All animal care, experiments and euthanasia were performed per protocols approved by the Chonnam National University Animal Research Committee. For the euthanasia of mice, mice were first rendered unconscious with isoflurane, and then death was confirmed after 5 to 10 min using carbon dioxide.
Immunofluorescence
Tumor tissue-mounted slides were subjected to a graded ethanol series for permeabilization, involving sequential immersion in 100%, 90%, 80%, 70%, and 60% ethanol for 5 min each. Antigen retrieval was then performed using citrate buffer (pH 6.0) for 15–20 min via heat-induced epitope retrieval (HIER). Slides were subsequently cooled under running water and treated with 0.1% Triton X-100 for 10 min at room temperature to reduce non-specific background staining. After three washes in PBS (5 min each), endogenous peroxidase activity was blocked using Endoblocker (Peroxidase-Blocking Solution, cat. no. S2023; Dako, Glostrup, Denmark) for 20 min at room temperature. Slides were again washed with PBS (3 × 5 min), and then incubated overnight at 4 °C with the primary antibody against Ki-67 (cat. no. ab16667; Abcam, Cambridge, UK), diluted 1:300 in blocking buffer. The following day, after three additional PBS washes, the sections were incubated with the secondary antibody, anti-rabbit Alexa Fluor 568 (cat. no. A1101; Invitrogen, California, USA), diluted 1:200, for 1 h at room temperature. Nuclear counterstaining was performed using DAPI (1:500 dilution; cat. no. D1306; Life Technologies, California, USA) for 10 min. After a final PBS wash (3 × 5 min), slides were mounted using Paramount Aqueous Mounting Medium (cat. no. S3025; Dako, Life Technologies, California, USA) and covered with a coverslip. Mounted slides were allowed to dry at room temperature for 24 h. Fluorescence images were acquired using the EVOS FL Imaging System (Invitrogen, California, USA). The number of Ki-67–positive cell per 100 tumor cells was used to determine the Ki-67 labeling proliferation index (%).
Statistical analysis
The significance of experimental differences was assessed using an unpaired Student’s t-test. Data are presented as mean ± standard error. All experimental assays were conducted independently in triplicate. Statistical analyses were conducted using SPSS version 21.0 (IBM Corp.). A p-value of < 0.05 was considered statistically significant.
Continue Reading
-

Julie Joubert captures the French Foreign Legion’s new recruits
“Patria Nostra”—which means “our homeland”—continuously subverts the stereotype of a soldier and the hypermasculinity of militarized bodies with quiet moments of grace and intimacy. Her camera shifts between group scenes of drills to…
Continue Reading