Google’s Gemini AI is set to make one of the most tedious office tasks a thing of the past — manually turning lengthy documents into slide decks. The company has introduced a new feature within its Gemini Canvas tool that can automatically…
Blog
-

Air Liquide expands its presence in India with the acquisition of NovaAir
Air Liquide announces today that it has entered into an agreement to acquire NovaAir, a leading industrial gas producer and supplier in India, from PAG, an Asia-focused private equity firm. This acquisition represents another investment for the Group in the country and a strategic milestone in its Indian growth story.
Founded in 2019, NovaAir is a full-service industrial gases platform supplying bulk industrial gases, critical specialty gases, and providing onsite services and EPC (Engineering, Procurement and Construction) support to its clients in the key industrial regions of East and South India. The company serves customers in the steel, automotive, fabrication, electronics, photovoltaic and healthcare industries, among others.
The acquisition of NovaAir which is present in the East and South of India complements Air Liquide’s existing operations in the North and West of the country, bringing a large project portfolio managed by an experienced team. Through this operation, the expanded geographical reach will substantially strengthen Air Liquide’s footprint in the industrial merchant market, allowing it to better serve customers in the growing automotive, metals, electronics and healthcare sectors.
This acquisition follows a series of recent investments in India by Air Liquide, underscoring the Group’s commitment to investing in the long-term growth opportunities of the Indian market. Air Liquide has been present in India since 1992 and has solid ambitions in this country. The Group’s operations in India encompass the supply of industrial and medical gases, engineering and construction services, cryogenic equipment manufacturing, medical systems for healthcare and the development of speciality chemical ingredients.
Emilie Mouren-Renouard, member of Air Liquide’s Executive Committee, notably in charge of supervising activities in India, stated:
“This acquisition represents a new step in our development in India. It demonstrates our long-term commitment to supporting the country’s industrial and healthcare development. This acquisition will significantly enhance our capacity to serve customers from small and medium enterprises to large industrial players, across a wider geographic footprint. It is another illustration of the growth potential of the Group.”
Continue Reading
-
Samsung’s first trifold phone may not unfold in the US
Samsung’s first trifold Galaxy has been detailed several times already, with leaked One UI 8.5 builds revealing many of its features. With the Galaxy XR headset unveiling behind it, Samsung should now be gearing up to take the wraps off its first…
Continue Reading
-

Pakistan submits ‘final position’ to Taliban as talks in Turkey enter third day – Firstpost
In their talks in Turkey, Pakistan has submitted its ‘final position’ to the Taliban and sought verifiable action against Tehrik-i-Taliban Pakistan (TTP). The Taliban have asked Pakistan to respect Afghanistan’s borders and not allow…
Continue Reading
-
Radio Pakistan Podcast I Kashmir Black Day I Amjad Iqbal (Senior Journalist) – RADIO PAKISTAN
- Radio Pakistan Podcast I Kashmir Black Day I Amjad Iqbal (Senior Journalist) RADIO PAKISTAN
- On Black Day, president, PM reaffirm support for Kashmiris Dawn
- Pakistan observes Kashmir Black Day to protest Indian occupation of IIOJK The Express…
Continue Reading
-

Exceptional new Pixel Watch 3 deal will make you feel like Christmas has come early this year
Released a little too late to top many holiday shopping lists this year, Google’s newest in-house smartwatch has another big problem… apart from all the obvious similarities with…Continue Reading
-

WFW advises Standard Chartered and BOI on Air India US$215m aircraft financing
Watson Farley & Williams (“WFW”) advised Standard Chartered Bank (“Standard Chartered”) and Bank of India, IFSC Banking unit (“BOI”), GIFT City, on the US$215m refinancing of six Boeing B777-300ER aircraft for Air India Limited (“Air India”).
Implementing an innovative structure, the first of its kind in India, this transaction marks the first time a financing for an Indian airline has been structured directly to a borrower in GIFT City, India’s emerging international financial hub, which has not been structured through Ireland.
Lending directly to AI Fleet Services IFSC Limited (“AI Fleet”) – Air India’s GIFT City-based subsidiary – demonstrates significant increase in market confidence and regulatory maturity in India. BOI’s involvement as lender establishes Indian domestic appetite for financing its aircraft and is evidence of the growing role of Indian domestic financial institutions are sure to play in India’s ambitions in aviation. This innovative structure not only streamlines execution but also facilitates Indian domestic participation in a more cost-effective structure.
Standard Chartered is a leading international banking group offering global financial services, whilst BOI is a major public sector bank in India providing a wide range of domestic and international banking solutions.
The cross-border WFW Aviation team that advised Standard Chartered and BOI was led by Singapore Asset and Structured Finance Partner Richard Williams, with outstanding support from Associates May Eng and Rheya Panjwani and Paralegal Lydia Ong. New York law advice was provided by Counsel Maxi Adamski-De Visser and Associate Chloe Sucato.
Richard commented: “The groundbreaking new structure used to complete this transaction sets a precedent for aviation finance for India. Lending directly to AI Fleet with the involvement of BOI represents a strong vote of confidence in Indian aviation and the recently developed regulatory framework in India. Advising on matters of this nature highlights WFW’s market leading footprint in Indian aviation finance. Our thanks go to Standard Chartered, BOI, Air India and AI Fleet for their confidence in us and the other legal professional advisors, whose collaborative approach was essential in closing this transaction”.
Continue Reading
-

Dow Jones Top Company Headlines at 3 AM ET: Novartis Agrees to Acquire Avidity Biosciences for $12 Billion | Corruption … – Morningstar
- Dow Jones Top Company Headlines at 3 AM ET: Novartis Agrees to Acquire Avidity Biosciences for $12 Billion | Corruption … Morningstar
- RNA STOCK ALERT: HALPER SADEH LLC IS INVESTIGATING WHETHER GlobeNewswire
- Novartis to Boost Neuroscience Portfolio With $12 Billion Deal for Avidity Biosciences MarketScreener
- Novartis Bets Big On Avidity Biosciences For Neuromuscular Pipeline Finimize
- Novartis Said to Near $70-Per-Share-Plus Deal for Avidity Bloomberg.com
Continue Reading
-
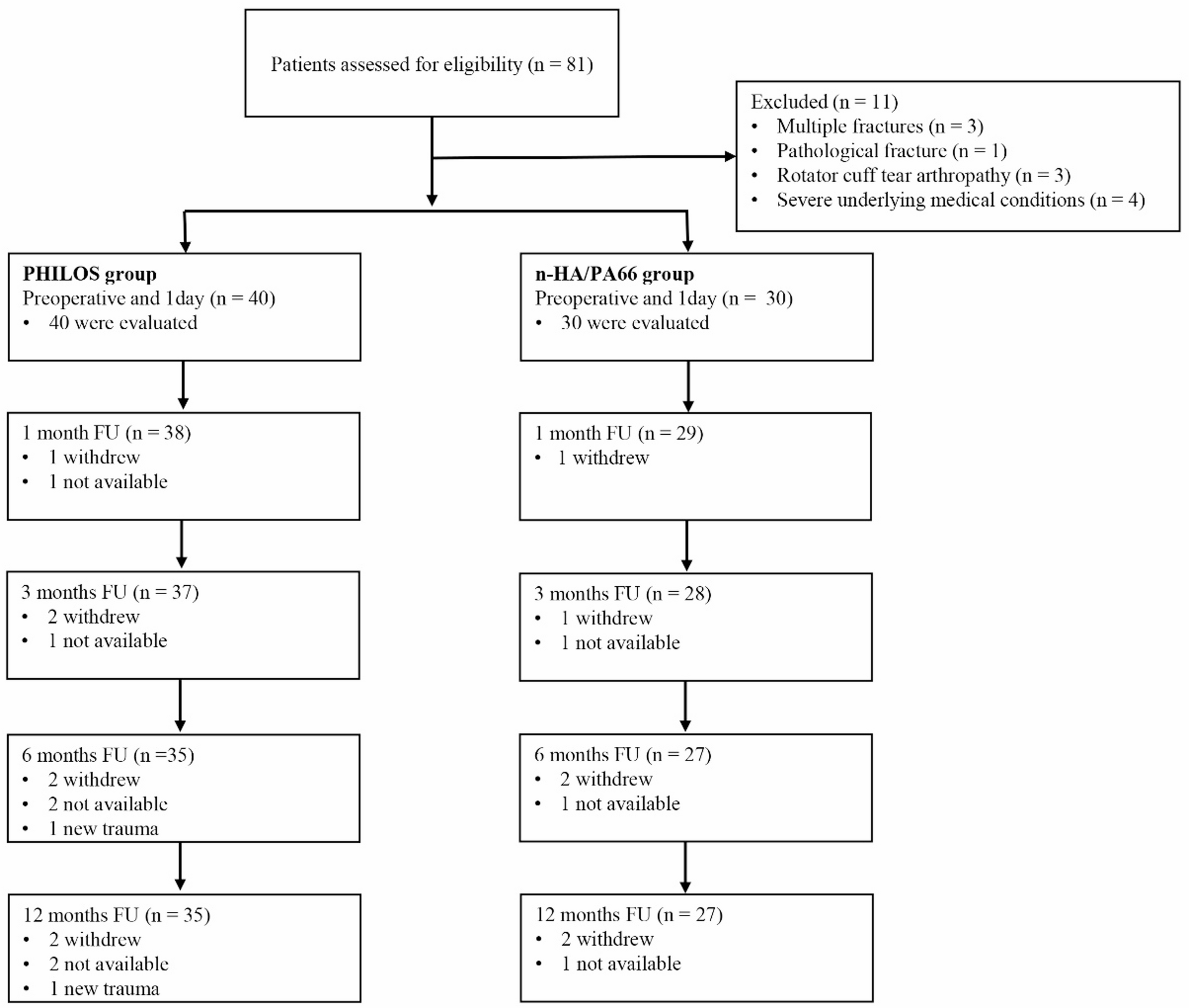
Efficacy of PHILOS combined with n-HA/PA66 augmentation for treating three- or four-part proximal humeral fractures in elderly patients | Journal of Orthopaedic Surgery and Research
This study retrospectively compared the clinical outcomes of 62 elderly patients with Neer three- or four-part proximal humeral fractures who underwent PHILOS plate fixation, with or without the adjunctive use of a bionic n-HA/PA66 composite for medial support.
Elderly patients who present with Neer three- or four-part proximal humerus fractures frequently exhibit significant comminution and severe osteoporosis, which can substantially complicate fracture reduction and fixation during surgical intervention [23]. A key difficulty in the surgical management of these fractures lies in obtaining precise reduction and strong fixation, with accurate reduction of the humeral head being paramount. Furthermore, the importance of a stable calcar to prevent secondary varus displacement, particularly in distracted varus-type fractures, is well-established [9, 10, 24, 25]. This study presents an innovative application of n-HA/PA66 for medial calcar support. After a one-year follow-up, the n-HA/PA66 group exhibited significantly smaller changes in NSA (3.6° ± 1.6°) and HHH (2.4 mm ± 0.7 mm) compared to the PHILOS group, which showed changes of 9.5° ± 2.0° and 4.1 mm ± 0.6 mm, respectively, indicating superior clinical outcomes. This advantage likely stems from the critical role of optimal reduction and minimized postoperative displacement in achieving favorable shoulder function, as suboptimal HHH and NSA can cause pain and rotator cuff weakness, directly impairing joint function. Consequently, at the final follow-up, patients treated with n-HA/PA66 demonstrated statistically superior functional outcomes, as reflected by their DASH (25.5 ± 5.1 points) and ASES (78.9 ± 8.9 points) scores, compared to the PHILOS group, which had DASH (28.1 ± 4.7 points) and ASES (73.0 ± 10.3 points) scores. However, long-term observations revealed no significant differences in CMS or range of motion between the two groups. This discrepancy may be attributed to the inherent subjectivity of the DASH and ASES scores, which emphasize overall postoperative functional recovery and pain perception. In contrast, the CMS and range-of-motion assessments provide a more objective evaluation of local shoulder function.
Double-plate osteosynthesis, typically employing a lateral locking plate in conjunction with a ventrally placed one-third tubular plate, has emerged as a technique to enhance primary stability and facilitate anatomical reconstruction in humeral head fractures characterized by calcar region destruction, aiming to prevent secondary dislocation [26]. The supplementary steel plate stabilizes the medial column of the humerus, thereby preventing displacement during the reduction process [27]. Theopold et al. [28] published a small case series describing their technique using a lateral locking plate combined with an inverted one-third tubular plate placed in the bicipital groove, with a mean follow-up of 24 months. The average CMS was 80 points, but the incidence of AVN was 14.3%. Mara Warnhoff et al. [29] conducted a retrospective review of 31 patients who underwent double-plate internal fixation, with an average follow-up of 30.9 months. At final follow-up, the mean CMS was 77 points, the ASES score was 76 points, and the NSA was 135° ± 13°. However, the study reported an AVN complication rate of 9.7%. Our results indicate that, at final follow-up, patients with n-HA/PA66 implants demonstrated a CMS of 73.8 ± 8.1 and an NSA of 132.7° ± 13.8°. Humeral head necrosis occurred in one patient (3.7%). Although our findings align with previous studies regarding functional and radiological outcomes, a key consideration is the surgical technique. The use of supplementary plates requires more extensive dissection and exposure, potentially increasing the risk of vascular and nerve bundle injury, as well as humeral head necrosis.
While segmental fibular allograft augmentation, placed endosteally in conjunction with a lateral locking plate, is frequently suggested as a strategy for enhancing medial support in proximal humerus fractures [14], the evidence regarding its efficacy remains somewhat equivocal. Dasari et al. [30] synthesized data from ten observational studies encompassing 802 patients, reporting a 95% rate of improved radiographic outcomes, increased ASES clinical outcome scores, and reduced odds of major complications in patients treated with a PHILOS plate augmented with a fibular allograft compared with those treated with a locking compression plate alone. However, these findings are not universally supported. Lee et al. [31] demonstrated that locking plate fixation combined with a fibular strut allograft yields satisfactory short-term outcomes in terms of humeral head support and maintenance of reduction. However, in their study, the CMS scores of the two groups showed no statistically significant difference (83.5 ± 6.5 and 87.8 ± 5.6), both of which were higher than our results (73.8 ± 8.1 and 70.5 ± 6.8). One possible explanation is that satisfactory therapeutic outcomes in their study were achieved with locking plates alone, making the supportive role of the fibular strut allograft non-additive. Furthermore, a randomized controlled trial [32] concluded that fibular allograft augmentation provided no additional benefit in treating medial column comminuted proximal humeral fractures, either radiographically or functionally. Variations in these study results may be attributed to several factors, including patient age, fracture type, graft- and technique-related variables, and methodological differences in study design and evaluation.
Moreover, some researchers have explored the use of bone cement to augment the stability of the PHILOS plate in proximal humeral fractures, reporting promising clinical outcomes and reduced complication rates [33], but the body of research on this technique remains limited. Furthermore, the potential for cement-related thermal necrosis warrants continued consideration.
Research demonstrates that the mean operative time for two-part proximal humeral fractures typically ranges between 60 and 90 min [25, 34]. In contrast, several studies have reported that the average surgical duration for three- and four-part proximal humeral fractures extends from 110 to 130 min [35, 36]. A primary factor contributing to the prolonged operative time in more complex fractures is the increased difficulty in achieving effective temporary fixation. In the present study, a comparison of operative durations between two fixation techniques revealed that the n-HA/PA66 group experienced an average reduction of 25 min compared to the PHILOS group. Our experience suggests that incorporating n-HA/PA66 to support the humeral head, followed by K-wire insertion through the n-HA/PA66 construct (as shown in Fig. 4B), can enhance the overall stability of temporary fixation. The n-HA/PA66 effectively acts as a bridging scaffold, reducing the need for repeated K-wire insertions and maintaining fracture reduction. The PHILOS combined with the n-HA/PA66 augmentation provides surgeons with a more dependable and efficient solution for treating complex proximal humeral fractures. Extended operative time is associated with an increased risk of complications [28, 32, 35]. Although this study did not find a statistically significant difference in complication rates between the two groups, nor did the use of implants appear to increase the incidence of related complications, these findings may be influenced by the study’s limited sample size and relatively short follow-up duration.
Although n-HA/PA66 is recognized as a bioactive material [15,16,17], current evidence does not demonstrate osseointegration with the host bone. Theoretically, the potential for micromotion persists under extreme conditions. Moreover, as with all implantable devices, managing deep infections remains highly challenging once established. Extended follow-up periods are necessary to substantiate the long-term reliability of this material. It is recommended that quantitative CT analyses be employed to evaluate changes in bone density at the scaffold-bone interface, thereby enabling an objective assessment of the integration process. Furthermore, collaboration with biomechanical research laboratories is essential to systematically investigate n-HA/PA66 scaffolds with diverse geometrical configurations, porosities, and mechanical properties to determine experimental parameters that ensure sufficient load-bearing capacity. Existing research offers valuable frameworks for identifying optimal treatment strategies [37, 38]. Conducting a multicenter, prospective, randomized controlled trial comparing PHILOS combined with n-HA/PA66 scaffolds to PHILOS with autologous iliac crest bone grafts and PHILOS with allograft bone grafts would provide higher-level evidence to inform clinical practice.
Despite the need to acquire a comprehensive dataset encompassing both imaging and functional outcomes, this study is subject to several limitations. First, the retrospective design, which lacks randomization, inherently introduces the potential for selection bias and confounding variables. Second, while considerable effort was made to ensure appropriate patient selection, the statistical power of this single-center study remains relatively modest, potentially hindering the detection of subtle yet meaningful differences between the groups. Furthermore, a larger sample size is necessary to enable robust subgroup analyses. Third, the subjective nature of the DASH score may contribute to the discrepancies observed between it and the more objective CMS. Finally, the relatively short one-year follow-up period necessitates caution in drawing definitive conclusions regarding the long-term efficacy of the investigated treatment approach, suggesting the need for studies with extended follow-up durations.
Continue Reading
-

Apple quietly made MacBook Pro changes you’ll actually appreciate the next time it breaks
Apple has recently unveiled a new 14-inch MacBook Pro powered by the M5 chip. Apparently, the M5 isn’t the only upgrade to the device this time, as a detailed teardown reveals that…Continue Reading
