404
The webpage or article you tried to access is currently unavailable.
This often occurs because the page has been moved or does not exist anymore. However, don’t worry, you can look for the article or a related one on our website…
This often occurs because the page has been moved or does not exist anymore. However, don’t worry, you can look for the article or a related one on our website…
As of December 31, 2023, a cumulative total of 722 pediatric drug clinical trials had concluded—either completed or discontinued—in mainland China. The annual trends indicated an increase in the number of completed, discontinued, and overall concluded clinical trials, with average annual growth rates of 21.5%, 89.7%, and 19.7%, respectively (R²=0.486). (Fig. 2). In terms of the trial phases, there were relatively few trials in the early stages such as Phase I and Phase II. The majority of the trials were concentrated in later stages, specifically Phase III (285, 39.5%) and Phase IV (150, 20.1%). Meanwhile, 6.2% of the trials adopted adaptive seamless trial designs such as I/II or II/III. Regarding the types of trial drugs, chemical drugs accounted for the largest proportion (393, 54.4%), while Chinese medicines/natural drugs accounted for the smallest proportion (29, 4.0%). In terms of the age of participants, most trials recruited adolescents (524, 72.6%) and children (429, 59.4%), newborns (124, 17.2%) and infants (274, 40.0%) trials were relatively fewer. From the perspectives of sponsors and trial institutions, the majority of trials were conducted by domestic sponsors (463, 64.1%), and primarily involved investigator-initiated studies (IST) (459, 63.6%). The number of clinical trials conducted in children’s hospitals was relatively low (109, 15.1%), with the majority being conducted in adult hospitals, disease control centres, and other trial institutions (613, 84.9%) (Table 1).
The growing trend of pediatric drug clinical trials in China
According to the disease classification for trials in ICD-11, as presented in eFig.1 of the supplementary material, the majority of pediatric drug clinical trials in China currently focused on vaccines (134, 18.6%) and respiratory diseases (99, 13.7%). The completion rate was most pronounced in vaccine trials, accounting for 132 trials or 20.5% of the total. Conversely, oncology trials exhibited the highest discontinuation rate, with 22 trials representing 27.2% of the total. At the same time, as shown in Fig. 3, the discontinuation rate of oncology trials involving adolescent subjects is higher, and the completion rate of vaccine trials involving infants and children is higher.
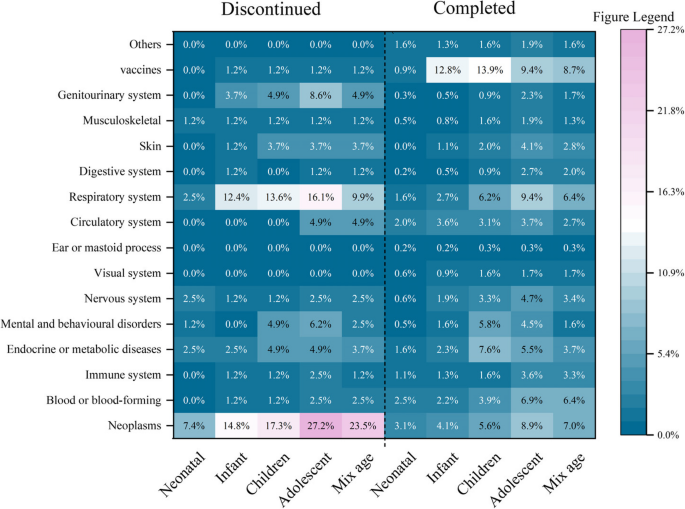
The proportion of diseases system in pediatric clinical trials among different age groups. Coded by International Classification of Diseases (ICD)−11 classification. Because most trials involved multiple age groups, the sum of the proportions for each age group exceeds the total
The Chinese government has successively released two editions of the “Rare Disease Drug List.” Consequently, we utilized this list, along with disease-related information, to screen clinical trials for rare diseases [20, 21]. Among the 722 trials, only 116 were for rare diseases, involving 34 types of rare disease drugs (eTable 1). The distribution of clinical trial leading institutions was shown in Fig. 4, primarily concentrated in the eastern regions of China, Such as North and East China, with 235 (30.3%) and 194 (30.3%) trials respectively. However, the discontinuation rate in these two regions was also high, with 37 (45.7%) and 25 (30.9%) trials discontinued respectively.
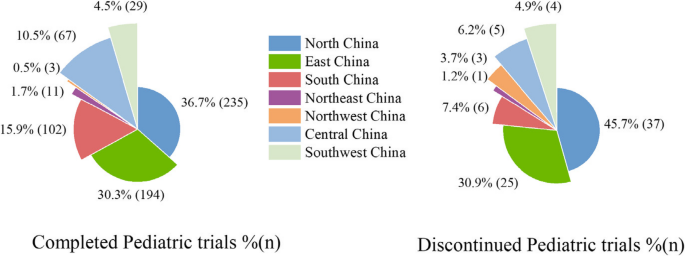
Geographical location percentage among Completed and Discontinued pediatric clinical trials. The completion/discontinuation percentage refers to the proportion of the number of completed or discontinued trials in a certain location to the total number of completed or discontinued trials
In terms of trial design, 504 (69.8%) trials adopted randomization, and 384 (53.2%) trials used blinding. The trial sample sizes were mostly in the range of 100–500 participants (259, 35.9%). Most pediatric drug clinical trials were parallel and active-medicine controlled trials, with fewer single-arm and placebo-controlled trials. The proportion of trials with DMCs and MRCT was relatively low (Table 2). This study compared trials with seamless designs such as I/II, II/III with conventionally designed trials such as phase I, II, and III (eTable 2). The results showed that these trials with seamless designs enrolled fewer participants and had stricter data requirements, and a total of 64.4% of the trials had DMCs [22].
A comparative analysis of completed and discontinued trials was presented in Tables 1 and 2, showing significant differences in multiple key dimensions (P < 0.05). In terms of trial phases, completed trials are concentrated in phase III (256, 39.9%) and phase IV trials (146, 22.8%), conducted by non-pediatric hospitals such as adult hospitals and disease control centres. Discontinued trials were primarily in phase III (29, 35.8%) and phase II (13, 16.1%), with a higher proportion conducted by pediatric hospitals. In terms of trial design, completed trials demonstrated higher standards in randomization, parallel design, and active-medicine control (P < 0.05), providing higher levels of evidence. In comparison, the level of evidence for discontinuing trials was lower, the enrollment was smaller, and higher proportions of trials with DMCs and MRCT.
A univariate subgroup analysis was performed on the duration of completed trials, excluding statistically insignificant variables such as sponsor type and trial design (eTable 3). The results of this analysis were presented in Fig. 5. Firstly, the duration of trials in China had gradually decreased over time(1201 days: 2003–2006, 458.5 days: 2019–2023). From the perspective of clinical trial stages, the seamless design trials of phases I/II, II/III, etc., took less time than the conventional trials with separate phases [23]. Additionally, there were significant differences in the trial duration among clinical trials of different diseases, with the duration of oncology clinical trials being the longest (1010 days) and that of vaccine clinical trials being the shortest (358.5 days). At the same time, the duration of trials with blinding, no adult participants, biological products, or domestic sponsors was shorter, and the trial duration of pediatric drug clinical trials with DMCs and MRCT was longer.
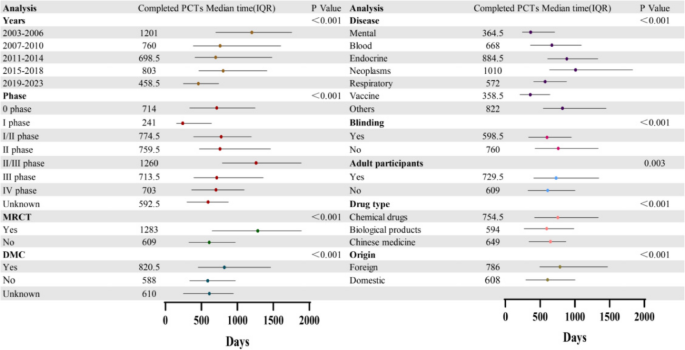
Duration of Completed pediatric drug clinical trials. Changes in the duration of trials were compared using time subsets of every four-year periods
The dependent variable was trial discontinuation or completion, and the independent variable was information related to the inclusion of trials. Following the exclusion of variables with P≥0.05 following univariate analysis (eTable 4), the final model was presented in Table 3. This refined model revealed that the number of enrolled participants, disease, control setting, and the establishment of DMCs are significantly correlated with the discontinued trials. Trials enrolling 0-50 participants had a significantly higher probability of discontinuation compared to trials with over 1,000 participants (OR = 17.286, 95%CI = 3.175-94.119, P = 0.001). The results also demonstrate that, compared with placebo-controlled trials, active-drug-controlled trials (OR = 0.278, 95%CI 0.110–0.707, P = 0.007) and uncontrolled trials (OR = 0.149, 95%CI 0.026–0.859, P = 0.033) are associated with a significantly lower probability of discontinuation. Additionally, trials with DMCs were more prone to discontinuation (OR = 4.054, 95%CI = 2.011–8.172, P < 0.001). Furthermore, the likelihood of discontinuation was significantly greater for respiratory disease trials (P < 0.001) and oncology clinical trials (P = 0.009) compared to trials for other diseases (Table 3).
Considering the reasons for trial discontinuation, we conducted a separate analysis of the significant variables in the previous logistic regression. As demonstrated in Table 4, the primary reasons for the discontinued trials were the safety or efficacy of the trial results (22, 27.2%) and commercial/trial plan adjustment(20, 24.6%). Small trials with fewer than 50 participants were discontinued because of plan adjustments (18, 27.6%). Placebo-controlled trials are more frequently discontinued due to safety or efficacy concerns than active-controlled or single-arm trials. Additionally, pediatric drug clinical trials involving adults were discontinued primarily due to trial results (17, 33.7%). In pediatric drug clinical trials for respiratory diseases and pediatric oncology, plan adjustments were the primary reasons for trial discontinuation, accounting for 4 (21.1%) and 8 (36.4%) respectively.

The naked mole rat is famous for living nearly 10x longer than other mammals of similar size.
| Photo Credit: Tim Evanson
The naked mole rat…
If Stardew Valley isn’t quite whimsical or magical enough for you as it is, then I have some great news.
Have you ever been half way through a new Stardew Valley playthrough and thought to yourself “hey, this is cool, but I wish I could shoot…

Saint Laurent’s slingback streak continued in London, this time on Tessa Thompson. The actor arrived at the BFI London Film Festival’s Academy Party in the house’s Loulou pumps, a $6,600 design defined by its elongated…

The MAS uses the exchange rate as its policy tool to curb inflation and support growth, given Singapore’s trade-dependent economy. Separately, Singapore will also release its September nonoil domestic exports data on Friday.
Malaysia
Malaysia will release third-quarter advance GDP figures and September trade data. Economic growth likely slowed to 4.3% in the third quarter from 4.4% in the previous quarter, as leading indicators point to weaker private consumption and softer imports of consumer goods, ANZ economists said.
While business approvals rose sharply in the first half, sentiment weakened in the third quarter across manufacturing and services, and slower capital goods imports suggest softer investment growth. Nominal imports fell faster than exports, likely improving net export slightly, though external demand remains subdued, ANZ added. The bank maintained its full-year growth forecast at 4.3%.
Malaysia's export growth may remain subdued in September amid global uncertainty and U.S. tariffs imposed in August, said TA Securities analyst Farid Burhanuddin. However, Malaysia's diversified export base, particularly strong trade ties with Asean, China, and other emerging markets, should help offset weaker U.S. demand, he added.
India
India will release inflation data on Monday, which is widely expected to show that price growth has resumed cooling after an uptick in August. A CPI print below the central bank's target could fuel expectations for more rate cuts ahead.
DBS economists forecast headline inflation to have eased to 1.5% on year in September from 2.1% the month before, taking the quarterly average slightly below the RBI's projected 1.8%.
"Global energy prices have also been subdued, offsetting the spillover risks from a weak rupee, while precious metals continue to stay buoyant," they said.
Wholesale price index data on Tuesday will round out the inflation picture. On Wednesday, attention will turn to trade figures for September, as India remains in talks with the U.S. over tariffs. DBS economists expect exports to moderate, leaving the trade deficit wide at $24 billion.
Any references to days are in local times.
Write to Emese Bartha at emese.bartha@wsj.com and Jihye Lee at jihye.lee@wsj.com
(END) Dow Jones Newswires
October 12, 2025 20:14 ET (00:14 GMT)
Copyright (c) 2025 Dow Jones & Company, Inc.

Photo: CFP
The UN humanitarian scale-up in the Gaza Strip is well underway, with cooking gas entering Gaza for the first time since March, the United Nations Office for the Coordination of Humanitarian Affairs (OCHA) said on Sunday.
More tents…
COVID-19 infection causes changes to sperm in mice that may increase anxiety in their offspring, a study released Saturday said, suggesting the pandemic’s possibly long-lasting effects on future generations.
Researchers at the Florey…

Hubert Hurkacz and Iga Swiatek are running it back. Two-time defending finalists Poland are the first team to commit to the 2026 United Cup.
The 2025 Wimbledon champion and former ATP World No. 6’s early commitment…

LONDON, Oct 13 (Reuters) – Spanish energy company Moeve has become the first external supplier of sustainable aviation fuel to join Shell’s blockchain-based platform for scaling SAF use, the oil major told Reuters after a deal was signed.
Shell’s Avelia platform is a “book and claim” system that aims to connect airlines, fuel suppliers and corporate buyers. Avelia, launched in 2022 with Amex Global Business Travel and Accenture, had facilitated over 41 million gallons of SAF use across 17 airports as of mid-2025.
Sign up here.
Moeve produces SAF from used cooking oil at its La Rábida Energy Park. It plans to expand overall capacity to 800,000 metric tons a year by 2030.
Reporting by Stephanie Kelly; Editing by Kirsten Donovan
Our Standards: The Thomson Reuters Trust Principles.