Afghan refugees board into trucks as they prepare to leave for their homeland through a border crossing point which partially opens following Pakistan Afghanistan ceasefire truce, at a camp in Chaman, a border town on the Pakistan Afghan border,…
Blog
-

MPs move to lodge parliamentary motion to strip Prince Andrew of dukedom | Prince Andrew
MPs have moved to lodge a parliamentary motion to strip Prince Andrew of his dukedom, in a rarely permitted move in the Commons.
The government is facing mounting pressure over the prince’s residence in the 30-room Royal Lodge in Windsor, where…
Continue Reading
-

Rheumatoid Arthritis and Fibromyalgia syndrome: A Bibliometric and Bio
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease characterized by systemic symmetrical polyarticular swelling, pain, and stiffness.1 Fibromyalgia syndrome (FMS) is a chronic rheumatic disorder that manifests as widespread…
Continue Reading
-

Hong Kong Philharmonic offer precision and poise in Seoul
At the Seoul Arts Center, Lio Kuokman and the Hong Kong Philharmonic set out their stall with two crisp modern postcards before turning to Tchaikovsky’s big‑ticket pairing of the First Piano Concerto and the Fifth Symphony. Unsuk Chin’s…
Continue Reading
-

Watch Oscar Isaac Try To Cover Tears for Fears on Bagpipe on ‘Fallon’
The actor also discussed being inspired by Mick Jagger and Prince for his performance in Guillermo del Toro’s Frankenstein
Oscar Isaac showcased his musical talents on The Tonight Show last night, performing Tears for…
Continue Reading
-
ATHS 2025 Conference – Addiction, Drug Use, Hepatitis, HIV
The International Congress on Addictology takes place at the Bellevue Conference Center in Biarritz, France.
ATHS 2025 brings together healthcare professionals, users, researchers, and experts to discuss key issues related to addiction,…
Continue Reading
-
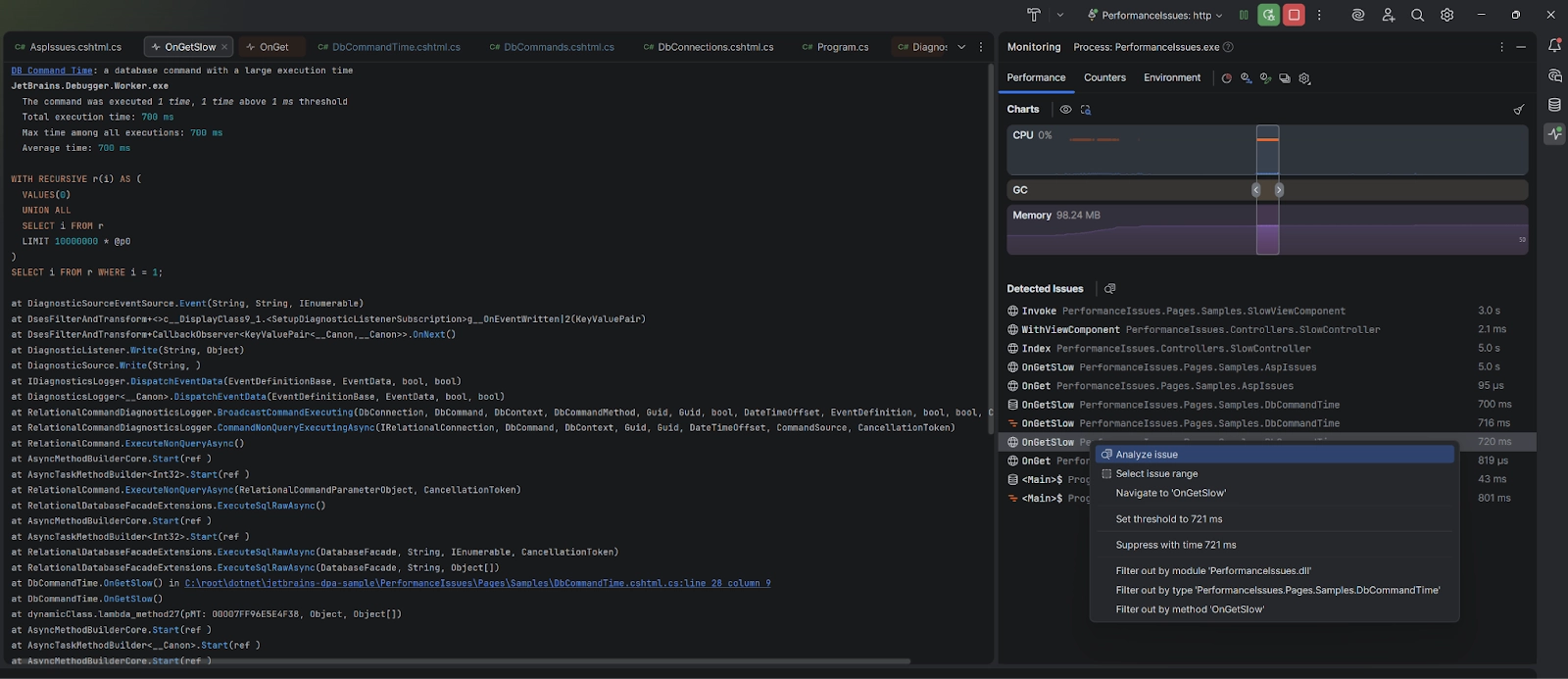
JetBrains brings more monitoring to IDE Rider EAP
JetBrains has released Rider 2025.3 EAP 6 with expanded monitoring capabilities for databases and ASP.NET. The Monitoring tool window now automatically detects slow queries and performance bottlenecks in real time, in…
Continue Reading
-

Sleep Fragmentation in TcMAC21 Mouse Model of Downs Syndrome
Jacob Tusk,1,* Marina Antonia Salinas Canas,1,* Tarik F Haydar,1,2 Terry Dean1,3
1Center for Neuroscience Research, Children’s National Hospital, Washington, DC, 20010, USA; 2Department of Anatomy and Neurobiology, Boston University Chobanian and Avedisian School of Medicine, Boston, MA, 02118, USA; 3Department of Critical Care Medicine, Children’s National Hospital, Washington, DC, 20010, USA
Correspondence: Terry Dean, Center for Neuroscience Research, Children’s National Hospital, Washington, DC, 20010, USA, Tel +1-202-476-6817, Email [email protected]
Background / Objective: Down syndrome (DS) is the most common chromosomal disorder worldwide, and approximately ¾ of individuals with DS demonstrate multifactorial sleep disturbances, including sleep apnea. As the effects of chromosome 21 triplication are complex, mouse models may provide valuable insights into the causal mechanisms of disordered sleep in DS. Although the recently developed transchromosomic TcMAC21 mouse model offers the closest genetic similarity to human DS, its sleep-wake architecture is unexplored. We hypothesized that TcMAC21 mice would exhibit sleep disruption similar to human DS, specifically with increased wakefulness and sleep fragmentation compared to the euploid controls.
Methods: Using a non-invasive piezo-electric sleep recording system, we evaluated the sleep-wake architecture in male TcMAC21 (TS, n=9) and euploid (EU, n=9) male control mice under a 12-hour light/dark cycle. Analyzed metrics included: total sleep percentage, bout frequency, and bout length.
Results: Compared to EU controls, TS mice exhibited a significant reduction in sleep bout duration (− 29.0%, p = 0.02) during the dark phase, with primary effect during the first 8 hours, culminating in an overall decrease in total sleep percentage (− 24.2%, p = 0.04). The light phase did not demonstrate statistically significant changes in total sleep percentage or sleep architecture.
Conclusion: TcMAC21 mice demonstrated significant sleep fragmentation during the dark phase, potentially reproducing some aspects of sleep disruption in Down syndrome. Interestingly, these findings differed from descriptions of sleep in other DS animal models. Given the high degree of DS gene replication and non-mosaic nature of the TcMAC21 model, it may provide unique insight into the neurologic and anatomic mechanisms of sleep dysfunction in Down syndrome.Introduction
Down syndrome (DS) is the most common chromosomal abnormality in humans and is caused by the presence of an extra copy of human chromosome 21 (Hsa21). This triplication leads to a multitude of clinical manifestations, including sleep disturbances. Sleep disorders in DS are common and multifactorial, with contributors such as obstructive and central sleep apnea and circadian dysrhythmia. Ultimately, DS patients experience poor sleep efficiency and excessive daytime sleepiness.1–6
Sleep architecture has been studied in multiple mouse models of DS that bear triplication of mouse chromosome regions that are syntenic to Hsa21. While triplication of mouse chromosome 16 (Mmu16), which mirrors a portion of Hsa21, causes fetal lethality,7 other models each demonstrate a unique sleep phenotype. Dp168,9 mice, which include triplication of the entire Mmu16, exhibit decreased sleep in both the light and dark phases.8 Meanwhile, Ts65Dn mice,10 containing a partial Mmu16 and partial Mmu17, show reduced sleep primarily in the dark phase.11 Ts1Cje mice, with a shorter region of triplication on Mmu16, display no baseline sleep differences but delayed sleep rebound after deprivation.11 Finally, the transchromosomic Tc1 mouse,7 which includes a fragmented mosaic of human chromosome 21 (Hsa21) including about 75% of the protein coding genes, shows sleep fragmentation during the light phase and increased sleep latency during the light-dark transition.12 These diverse sleep phenotypes are thought to be driven by each model’s distinct genetic composition, highlighting the importance of accurately recapitulating the human disorder’s chromosomal abnormality in animal models.
The newly developed transchromosomic TcMAC2113 mouse model offers a significant advancement for DS research. It replicates 93% of the protein-coding genes on Hsa21q, including key genes associated with DS.13 Furthermore, unlike the mosaic nature of the Tc1 model,7 TcMAC21 is non-mosaic,13 ensuring uniform trisomic genetic material across cells and enhancing the reproducibility of phenotypes. However, the sleep architecture of TcMAC21 mice has not yet been described. Given its close genetic resemblance to human DS, this study aims to characterize sleep patterns in TcMAC21 mice to assess their potential as a platform for exploring the etiology and consequences of sleep disruptions in DS. We hypothesized that TcMAC21 mice would exhibit sleep disruption similar to human DS, specifically with increased wakefulness and sleep fragmentation compared to the euploid controls.
Materials and Methods
Mice
All procedures and experimental design were approved by the Institutional Animal Care and Use Committee at Children’s National Hospital (Protocol 30786) and follows National Institute of Health (NIH) and Animal Research: Reporting of In-Vivo Experiments (ARRIVE) guidelines. Our vivarium is maintained at 72°F ±2°F, and the humidity range is 30–50%. Mice had unrestricted access to standard laboratory diet, water, and nesting squares and were maintained under a 12-hour light/dark cycle. The TcMAC21 line was originally acquired from Jackson Laboratories (Strain 035561). As the TcMAC21 line has been reported to have a high variation of fecundity,14 which is anecdotally consistent with our experience, all TcMAC21 females were reserved for breeding purposes and not available for use in this study; a convenience sample of 10 male TcMAC21 (TS) mice and 10 male euploid (EU) littermates was used. Given the light/dark phase-specific findings noted on previous studies of DS mouse models,7–11 an a priori power analysis based on variability in murine sleep noted in our previous experience15 suggested that n=10 would provide adequate power to detect a 10% decrease in 12-hr total sleep time with 95% certainty, and would exceed the numbers in previous sleep reports in DS mouse models.11 Data were collected over 3 separate litters of mice with as many as 8 mice recorded at a time; each recording run included simultaneous recordings of mice of both genotypes, randomly assigned to sleep chambers within a light-tight, sound-proof cabinet (Actimetrics). The analyzed mice were 6.3 ± 1.2 weeks (TS) and 7.1 ± 0.9 weeks (EU) old at the time recording.
Sleep Recording and Processing
The use of a non-invasive sleep recording system limits the potential for surgery-related factors (eg recovery from anesthesia, wound healing, inflammatory response) to influence changes in sleep-wake behavior, which is a consideration in the TcMAC21 mice that bear a global transgenic modification. All mice underwent a 5–7 day acclimation period, during which they were individually housed in the piezo-electric chambers (Signal Solutions) with free access to food and water. Up to 8 chambers are housed within a circadian cabinet that would be used for non-invasive sleep recording. After the acclimation period, those chambers would then continuously collect motor activity for 48 hours without disruption. The activity thresholds for distinguishing sleep and wake states were determined using commercially available SleepStats 2020 (Signal Solutions), which has previously shown 89% sensitivity and 96% specificity for wake versus sleep (NREM + REM);16–18 however, this system’s sensitivities/specificities for distinguishing NREM and REM sleep are limited16 and were not utilized in this study. Data were exported as CSV files and analyzed for sleep-wake epochs, with checks for sensor errors or electrical interference. For each mouse, two consecutive 24-hour light/dark (L:D) cycles were analyzed, generating metrics including sleep bout length histograms and percent sleep. Of note, the TcMAC21 mice did bear distinguishable physical characteristics noted previously,13 including abnormal facies and shortened ears, making blinding investigators to mouse genotype impossible as the investigators interacting with the mice were the same as those collecting and analyzing the sleep data; however, the piezosleep sleep analysis system does not require user input to calculate each of these metrics, limiting the possible introduction of researcher bias in the analysis.
Data Analysis and Availability
Statistical analyses of comparisons were conducted using Prism (GraphPad version 10.1). Our primary outcomes of differences in total light and dark phase sleep chosen given the previous histories of finding light- and dark-specific changes in Down Syndrome mice.7–11 After applying Grubbs’ extreme studentized deviate testing (α=0.01) to the 24-hr and light/dark phase total sleep times, two mice were identified as statistical outliers and removed from all analyses: 1 EU that slept 43% less than the group average, 1 TS that slept 58% above the group average; at the time of the recordings no mice were observed to have been ill by appearance or gross motor behaviors. For the remaining mice (9 TS, 9 EU), no further outliers were removed from any analyses. Twenty-four hour, light, and dark total sleep percentages were analyzed via unpaired T-tests. Evaluation of sleep architecture (sleep percentage, sleep bout duration, sleep bout frequency) in 4-hour bins were conducted by two-way repeated measures ANOVA followed by comparisons between genotypes (EU vs TS) per bin; false discovery correction for multiple comparisons was conducted as per Benjamini-Krieger-Yekutieli procedure.19
Results
TcMAC21 (TS) mice exhibited a statistically significant reduction in 24-hour total sleep percentage relative to euploid (EU) controls (mean ± SD: 42.8 ± 8.3% and 50.1 ± 4.2%, respectively; p = 0.04; Figure 1A). This difference was accounted for primarily by a significant decrease in dark phase sleep (26.6 ± 6.3% vs 35.1 ± 9.6%, respectively; p = 0.041), with a non-significant reduction during the light phase (59.2 ± 12.5% vs 65.1 ± 5.6%; p = 0.21). Because a previous animal DS model demonstrated a sleep phenotype isolated to the first half of the dark phase,12 we next evaluated sleep metrics in 4-hour intervals (Figure 1A). For total sleep percentage, genotype demonstrated a significant effect (p=0.041) while time of day (p=0.10) and their interaction (p=0.62) did not. Pairwise comparisons identified decreased sleep during the first four hours of the dark phase (ZT 1200–1400; p=0.04), but ultimately none were statistically significant after correction for false discovery rate (Figure 1A). Analysis of the light phase suggested only significant effects of time of day (p<0.001) without an effect of genotype (p=0.21) or an interaction between genotype and time of day (p=0.84).
Figure 1 Comparison of sleep architecture in TS and EU mice. (A) (left) Sleep expressed as a percentage of total time for 24 hour and 12 hour periods. TS mice (magenta) show reduced sleep compared to EU controls (green) when measured over 24 hours, primarily due to a decrease during the dark phase (indicated by horizontal black bars). (right) Sleep expressed as a percentage of 4-hour intervals throughout the 24-hour day. TS mice showed trends towards reduced sleep during the first third of the dark phase (ZT 1200–1600). (B) Mean sleep bout duration for each 4-hour interval throughout the 24-hour day. TS mice (magenta) show reduced sleep bout duration sleep compared to EU controls (green) during the first 8 hours of the dark phase (ZT 1200–1600, ZT1600-1800). (C) Mean sleep bout frequency for each 4-hour interval throughout the 24-hour day. For all panels, Tukey box plots indicate median and interquartile range (IQR) (via box) and minimum/maximum up to 1.5x the IQR above/below the 25%ile and 75%ile, respectively (via whiskers); outliers represented by individual points. Individual ZT’s are marked the center of a four-hour interval. For all experiments, n = 9 for EU, n = 9 for TS. Any statistically significant P-values are detailed.
We next considered sleep bout duration and frequency in 4-hour bins to characterize the changes in sleep architecture underlying the observed sleep differences. Genotype (TS vs EU) produced a significant decrease in mean sleep bout duration during the dark phase (240.9 ± 81.5 vs 339.2 ± 80.5 s, respectively; p=0.02; Figure 1B), while the factors time of day (p=0.07) and their interaction (p=0.66) did not. Pairwise comparisons revealed significantly decreased mean sleep bout duration in TS mice during the first 8 hours of the dark period (ZT 1200–1600: 189.0 ± 66.0 vs 301.0 ± 108.7 s, p = 0.02; ZT 1600–2000: 214.0 ± 67.2 vs 340.1 ± 132.6 s, p=0.03), without an effect during the last 4 hours (ZT 2000–2400: 376.4 ± 124.7 vs 319.7 ± 207.2 s, p=0.49). Conversely, no significant effects were seen on mean bout length (Figure 1C) during the light phase (genotype p = 0.49, time of day p = 0.12, interaction p = 0.53). Similarly, no significant differences were found in the analyses of the sleep bout frequencies in either the dark or light phases (dark: time of day p = 0.29, genotype p = 0.76, interaction p = 0.59; light: time of day p = 0.45, genotype p = 0.67, interaction p = 0.33).
Discussion
The TcMAC21 (TS) mice demonstrated significant alterations in sleep-wake architecture, most notably driven by a decrease in sleep bout duration during the dark phase, the primary active period for mice. As no compensatory changes in bout frequency were observed during the dark phase, the degree of sleep loss over the 12 hours remained significant. This contrasts with sleep behavior during the primary resting (ie light) phase, which may have trended towards comparatively smaller decreases in overall sleep time and sleep bout duration, but did not reach statistical significance. When compared to other DS mouse models (ie Dp16, Ts65Dn, Ts1Cje, Tc1), the dark-phase-specific decrease in sleep of TcMAC21 mice most closely resembles the sleep behavior of the Ts65Dn model. However, Ts65Dn mice also exhibit an extended period of wakefulness during the first 6 hours of the dark phase, unlike the TcMAC21 mice slept for ~21% during ZT 1200–1600; they both demonstrated shortened sleep bouts when they did sleep during the dark phase. Tc1 mice do demonstrate sleep fragmentation similar to TcMAC21 mice, but the effects are primarily observed during the light phase. These differences highlight the variability in sleep phenotypes across DS mouse models, suggesting that sleep disturbances in trisomy 21 are likely polygenic in origin, with different combinations of triplicated genes contributing to varying phenotypic outcomes (summarized in Table 1).

Table 1 Comparison of Baseline Sleep Architecture Findings Between Mouse Models of Down Syndrome
The reduction in sleep bout length observed in TcMAC21 mice is consistent with sleep fragmentation. However, the exact cause of sleep fragmentation in these mice remains unknown. As human DS is associated with obstructive and central sleep apneas, it is possible that the TcMAC21 mice could be experiencing a similar phenomenon. We and previous reports of the TcMAC21 mice have noted changes in craniofacial development, including shorter and wider snouts,13 therefore a contribution of altered airway anatomy to disordered breathing during sleep is possible. We predict that future studies employing whole body plethysmography may determine the roles of obstructive or central hypoventilation to the dark phase disturbances we observed. Furthermore, simultaneous incorporation of polysomnography would also improve upon our system’s limitation being unable to differentiate NREM and REM sleep. There are at least two benefits of polysomnography in this mouse model. The first is that it will be necessary to determine if the shortened sleep bouts seen in the TcMAC21 mice prevent normal quantities of REM sleep, similar to human patients.20 Should the murine sleep behavior prove consistent with the human disorder, then the TcMAC21 mouse may be a useful platform for further investigation of the mechanisms of disordered sleep as well as testing new therapeutic strategies. The second is to provide increased sensitivity for changes in sleep that may have evaded detection by our piezoelectric system. For instance, we noted consistent albeit non-significant trends towards less total sleep percentages in the light phase at a smaller magnitude than our dark-phase findings. Use of polysomnography will provide a more definitive characterization of sleep-wake balance during that phase, determining if the sleep phenotypes are truly isolated to the dark phase.
The TcMAC21 mouse presents an opportunity to dissect the contributions of sleep disruption, itself, to the greater neurodevelopmental pathophysiology in DS. For instance, sleep disturbance in human DS is associated with impairments in expressive language development.21 The TcMAC21 model may be able to address the role of the sleep phenotype, itself, on communicative ultrasonic vocalizations.22 Similarly, the TcMAC21 mice demonstrate overexpression of amyloid precursor protein in the hippocampus as well as significant learning and memory deficits on behavioral testing,13 which may model the increased susceptibility for dementia in human DS.23 However, it is also known that sleep fragmentation, itself, may contribute to this type of pathophysiology.24,25 Using TcMAC21 to further investigate a causal role for sleep disruption in neurodegeneration in the human DS population provides an avenue for developing therapeutic strategies.
We are aware of several limitations of our study due to experimental design. At the time of our experiments, the TcMAC21 mice available for prolonged sleep studies were few and included only male mice of a limited age range, described above. Expanding the sample size would provide for more statistical power to detect subtle sleep phenotypes, while the inclusion of females will be important to provide insight into the causes of the subtle sex and age differences DS patients, including males demonstrating increased N1 sleep26 as well as increased daytime sleepiness and napping behaviors compared to females.27 Finally, we also note that the average ages of our cohorts were approximately 1 week apart, with the EU mice being older than TS mice. One study comprehensively examining the influence of age on murine sleep-wake behavior during murine adolescence (postnatal day 15 through P87) did not find significant differences in total 24 hour sleep28 with age, making it less likely that age, itself was a confounding factor in our primary outcome. Similarly, NREM and REM sleep episode durations reached adult levels at P25 and P41, respectively, making it less likely that age governed the observed differences in sleep bout duration. Nevertheless, the impact of TcMAC21 on age-related changes in sleep would be of importance to investigate, first during adolescence given the subtle changes in NREM-REM balance that are seen during the first few months of development,28 as well as during longer time intervals (~12 months) during which there are gross changes in sleep architecture, including increased sleep during the phase.29
Conclusion
By recapitulating 93% of protein-coding genes from human chromosome 21q13, the TcMAC21 model represents one of the most accurate transgenic models of DS. Interestingly, in contrast to human DS sleep behavior, the TcMAC21 mice demonstrated significant sleep fragmentation, resulting in substantially decreased sleep during the dark phase, the primary wake time for mice. This model offers a unique platform for further investigate DS-related sleep disturbances, including sleep apnea, neuronal control of sleep, and long-term neurodevelopmental outcomes.
Data Sharing Statement
All data are available from the corresponding author upon reasonable request in accordance with journal guidelines.
Acknowledgments
We would like to acknowledge Khristine Amber Pasion and Zeynep Atak for their invaluable assistance in maintaining the TcMAC21 colony which was used for this project.
Author Contributions
JT and MS were responsible for data curation, formal analysis, and writing the original draft. TD and TH were responsible for study conceptualization, funding acquisition, supervision, and writing – reviewing/editing. All authors gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
T.D. was supported by NINDS K08NS131529. T.H. was supported by R01NS116418 and R01NS136246.
Disclosure
All authors do not have any financial or non-financial relationships or conflicts of interest to disclose.
References
1. Fan Z, Ahn M, Roth HL, Li L, Vaughn BV. Sleep apnea and hypoventilation in patients with down syndrome: analysis of 144 polysomnogram studies. Children. 2017;4(7):55. doi:10.3390/children4070055
2. Lovos A, Bottrill K, Sakhon S, et al. Circadian sleep-activity rhythm across ages in down syndrome. Brain Sci. 2021;11(11):1403. doi:10.3390/brainsci11111403
3. Santos RA, Costa LH, Linhares RC, Pradella-Hallinan M, Coelho FMS, Oliveira GDP. Sleep disorders in down syndrome: a systematic review. Arq Neuropsiquiatr. 2022;80(4):424–443. doi:10.1590/0004-282x-anp-2021-0242
4. Lee CF, Lee CH, Hsueh WY, Lin MT, Kang KT. Prevalence of obstructive sleep apnea in children with down syndrome: a meta-analysis. J Clin Sleep Med. 2018;14(5):867–875. doi:10.5664/jcsm.7126
5. Horne RS, Shetty M, Vandeleur M, Davey MJ, Walter LM, Nixon GM. Assessing sleep in children with down syndrome: comparison of parental sleep diaries, actigraphy and polysomnography. Sleep Med. 2023;107:309–315. doi:10.1016/j.sleep.2023.05.003
6. Esbensen AJ, Hoffman EK, Beebe DW, Byars KC, Epstein J. Links between sleep and daytime behaviour problems in children with down syndrome. J Intellect Disabil Res. 2018;62(2):115–125. doi:10.1111/jir.12463
7. Vacano GN, Duval N, Patterson D. The use of mouse models for understanding the biology of down syndrome and aging. Curr Gerontol Geriatr Res. 2012;2012:717315. doi:10.1155/2012/717315
8. Levenga J, Peterson DJ, Cain P, Hoeffer CA. Sleep behavior and EEG oscillations in aged Dp(16)1Yey/+ mice: a down syndrome model. Neuroscience. 2018;376:117–126. doi:10.1016/j.neuroscience.2018.02.009
9. Takahashi T, Sakai N, Iwasaki T, Doyle TC, Mobley WC, Nishino S. Detailed evaluation of the upper airway in the Dp(16)1Yey mouse model of down syndrome. Sci Rep. 2020;10(1):21323. doi:10.1038/s41598-020-78278-2
10. Akeson EC, Lambert JP, Narayanswami S, Gardiner K, Bechtel LJ, Davisson MT. Ts65Dn — localization of the translocation breakpoint and trisomic gene content in a mouse model for down syndrome. Cytogenet Cell Genet. 2001;93(3–4):270–276. doi:10.1159/000056997
11. Colas D, Valletta JS, Takimoto-Kimura R, et al. Sleep and EEG features in genetic models of down syndrome. Neurobiol Dis. 2008;30(1):1–7. doi:10.1016/j.nbd.2007.07.014
12. Heise I, Fisher SP, Banks GT, et al. Sleep-like behavior and 24-h rhythm disruption in the Tc1 mouse model of down syndrome. Genes Brain Behav. 2015;14(2):209–216. doi:10.1111/gbb.12198
13. Kazuki Y, Gao FJ, Li Y, et al. A non-mosaic transchromosomic mouse model of down syndrome carrying the long arm of human chromosome 21. Elife. 2020;9. doi:10.7554/eLife.56223
14. Jackson laboratory website: STOCK Tc(HSA21, CAG-EGFP)1Yakaz/J. Available From: https://www.jax.org/strain/035561.
Accessed .October 2 , 202515. Dean T, Allen RP, O’Donnell CP, Earley CJ. The effects of dietary iron deprivation on murine circadian sleep architecture. Sleep Med. 2006;7(8):634–640. doi:10.1016/j.sleep.2006.07.002
16. Yaghouby F, Donohue KD, O’Hara BF, Sunderam S. Noninvasive dissection of mouse sleep using a piezoelectric motion sensor. J Neurosci Methods. 2016;259:90–100. doi:10.1016/j.jneumeth.2015.11.004
17. Mang GM, Nicod J, Emmenegger Y, Donohue KD, O’Hara BF, Franken P. Evaluation of a piezoelectric system as an alternative to electroencephalogram/ electromyogram recordings in mouse sleep studies. Sleep. 2014;37(8):1383–1392. doi:10.5665/sleep.3936
18. Donohue KD, Medonza DC, Crane ER, O’Hara BF. Assessment of a non-invasive high-throughput classifier for behaviours associated with sleep and wake in mice. Biomed Eng Online. 2008;7(1):14. doi:10.1186/1475-925X-7-14
19. B Y, K AM, Y D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93(3):491–507. doi:10.1093/biomet/93.3.491
20. Gaza K, Gustave J, Rani S, Strang A, Chidekel A. Polysomnographic characteristics and treatment modalities in a referred population of children with trisomy 21. Front Pediatr. 2022;10:1109011. doi:10.3389/fped.2022.1109011
21. Edgin JO, Tooley U, Demara B, Nyhuis C, Anand P, Spano G. Sleep disturbance and expressive language development in preschool-age children with down syndrome. Child Dev. 2015;86(6):1984–1998. doi:10.1111/cdev.12443
22. Premoli M, Pietropaolo S, Wohr M, Simola N, Bonini SA. Mouse and rat ultrasonic vocalizations in neuroscience and neuropharmacology: state of the art and future applications. Eur J Neurosci. 2023;57(12):2062–2096. doi:10.1111/ejn.15957
23. Fortea J, Zaman SH, Hartley S, Rafii MS, Head E, Carmona-Iragui M. Alzheimer’s disease associated with down syndrome: a genetic form of dementia. Lancet Neurol. 2021;20(11):930–942. doi:10.1016/S1474-4422(21)00245-3
24. Duncan MJ, Guerriero LE, Kohler K, et al. Chronic fragmentation of the daily sleep-wake rhythm increases amyloid-beta levels and neuroinflammation in the 3xTg-AD mouse model of alzheimer’s disease. Neuroscience. 2022;481:111–122. doi:10.1016/j.neuroscience.2021.11.042
25. Havekes R, Meerlo P, Abel T. Animal studies on the role of sleep in memory: from behavioral performance to molecular mechanisms. Curr Top Behav Neurosci. 2015;25:183–206.
26. Gimenez S, Videla L, Romero S, et al. Prevalence of sleep disorders in adults with down syndrome: a comparative study of self-reported, actigraphic, and polysomnographic findings. J Clin Sleep Med. 2018;14(10):1725–1733. doi:10.5664/jcsm.7382
27. Moriyama N, Sawatari H, Chishaki A, et al. 0772 age and sex impact on symptoms of sleep-disordered breathing in people with down syndrome -a nation-wide study In Japan. Sleep. 2018;41(Suppl_1):A287. doi:10.1093/sleep/zsy061.771
28. Nelson AB, Faraguna U, Zoltan JT, Tononi G, Cirelli C. Sleep patterns and homeostatic mechanisms in adolescent mice. Brain Sci. 2013;3(1):318–343. doi:10.3390/brainsci3010318
29. Soltani S, Chauvette S, Bukhtiyarova O, et al. Sleep-wake cycle in young and older mice. Front Syst Neurosci. 2019;13:51. doi:10.3389/fnsys.2019.00051
Continue Reading
-

Bird flu hiding in cheese? The surprising new discovery – Science Daily
- Bird flu hiding in cheese? The surprising new discovery Science Daily
- Bird flu virus survives in raw-milk cheese for months News-Medical
- Live Avian Flu Can Survive in Raw Milk Cheese Up to Six Months Food Poisoning Bulletin
- Bird flu virus can…
Continue Reading
-

Relationship Between Thyroid Hormones and Fat Distribution in Patients
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by insulin resistance, hyperglycaemia, and various metabolic abnormalities.1 Over the past few decades, the global prevalence of T2DM has continuously increased at an alarming rate, with estimates suggesting that more than 463 million people worldwide are currently living with diabetes, the vast majority of which are T2DM cases.2 This surge is largely driven by rising obesity rates, unhealthy lifestyles, and population aging.3 T2DM is not only a major cause of morbidity and mortality but also places a tremendous economic burden on healthcare systems due to the frequent development of complications, including cardiovascular disease, nephropathy, and neuropathy.4
Among the numerous complications associated with T2DM, abnormal fat distribution plays a pivotal role in the progression of the disease and the development of insulin resistance.5 Visceral fat is known to contribute significantly to conditions related to metabolic dysfunction, such as dyslipidaemia, hypertension, and inflammation. Subcutaneous fat, though less harmful than visceral fat, also can contribute to metabolic derangements when present in excess.6 Given the importance of fat distribution in the pathophysiology of T2DM, understanding the factors that influence fat storage and distribution is critical for the development of effective therapeutic interventions.7
Thyroid hormones are well-established regulators of metabolism and energy expenditure.8 These hormones, including free triiodothyronine (FT3) and free thyroxine (FT4), play key roles in lipid metabolism, thermogenesis, and glucose homeostasis. Thyroid-stimulating hormone (TSH), which is produced by the pituitary gland, regulates the synthesis and secretion of both FT3 and FT4.9,10 In clinical practice, thyroid function is typically evaluated by measuring thyroid hormone levels, with deviations from normal levels indicating either hypo- or hyperthyroidism.11,12 Interestingly, research has demonstrated that even in euthyroid individuals (those with normal thyroid function), variations in thyroid hormone levels may have significant metabolic consequences.13,14
Several studies15–17 have explored the interplay between thyroid hormones and fat distribution, particularly in relation to obesity and metabolic syndrome. One study18 found that individuals with elevated FT3 levels tend to have higher body fat percentages, particularly involving visceral fat, which is associated with greater risks of insulin resistance and cardiovascular diseases. Conversely, higher FT4 levels are linked to lower body fat and improved metabolic profiles, suggesting a protective role of FT4 in fat metabolism. These findings are consistent with the known metabolic effects of thyroid hormones, with FT3 being the more metabolically active hormone that increases the basal metabolic rate and promotes lipid oxidation.19
However, the relationship between thyroid hormone levels and fat distribution in T2DM patients remains poorly understood. Most studies of this relationship have focused on general populations or individuals with obesity, without specifically examining T2DM patients. Given the unique metabolic characteristics of T2DM patients, including insulin resistance and altered glucose metabolism, it is important to investigate how thyroid hormone levels influence fat distribution in this population.
The present study aimed to elucidate the relationship between thyroid hormones (FT3, FT4, and TSH) and fat distribution, specifically in terms of visceral fat area (VFA) and subcutaneous fat area (SFA), in T2DM patients with normal thyroid function. By characterizing these relationships, a goal of this study was to provide insight into the potential roles of thyroid hormones in the pathophysiology of T2DM and its complications. This information, in turn, can support the development of targeted treatments for metabolic management in T2DM patients.
Materials and Methods
Study Design and Participants
This cross-sectional study included 2,956 T2DM patients recruited from a tertiary hospital between 2021 and 2023. The initial dataset contained 10,416 entries. The inclusion criteria were: (1) age ≥18 years, (2) confirmed diagnosis of T2DM according to the World Health Organization (WHO) criteria, and (3) availability of complete data for VFA, SFA, and thyroid hormone levels (FT3, FT4, and TSH). The exclusion criteria were applied as follows: duplicate entries (6,237 cases), missing age or age <18 years (7 cases), incomplete VFA or SFA data (875 cases), unconfirmed T2DM diagnosis (31 cases), presence of thyroid disease (122 cases), and missing FT3/FT4/TSH data (188 cases). After these exclusions, 2,956 patients with complete data were included in the final analysis.
The study was approved by the Institutional Review Board of the hospital, and written informed consent was obtained from all participants prior to enrolment.
Data Collection
Demographic and Clinical Characteristics
Baseline demographic and clinical data were collected from medical records and included: age, gender, body mass index (BMI), and waist-to-hip ratio (WHR); blood pressure (systolic and diastolic); and lifestyle factors such as smoking status (never, quit, occasionally, or daily) and alcohol consumption (never, quit, occasionally, or daily).
Thyroid Hormone Measurements
Fasting blood samples were obtained in the morning from all participants for the measurement of thyroid hormone levels.20 Serum FT3, FT4, and TSH levels were measured using enzyme-linked immunosorbent assays (ELISAs). To explore differences in fat distribution across varying levels of thyroid hormones, we stratified participants into three groups (low, normal, and high), based on serum FT3, FT4, and TSH levels. The cutoffs for these categories were defined according to standard clinical reference ranges used in routine laboratory practice and prior studies on thyroid function in euthyroid individuals: FT3 (low <1.8, normal 1.8–3.8, high >3.8 pg/mL), FT4 (low <0.78, normal 0.78–1.86, high >1.86 ng/dL), and TSH (low <0.38, normal 0.38–5.57, high >5.57 mU/L). These ranges were chosen to ensure consistency with clinical definitions and to assess potential trends across the spectrum of thyroid hormone concentrations within the euthyroid range. Quality control procedures were applied to ensure measurement accuracy, and extreme values were addressed based on statistical feedback. Specifically, values for creatinine (Cr), FT4, and TSH were adjusted as follows: Cr (151.72 μmol/L and 103.9 μmol/L), FT4 (7.71 ng/dL), and TSH (97.743 mU/L). These adjustments corrected data entry inconsistencies or biologically implausible values, improving data integrity and minimizing potential bias. Sensitivity analyses indicated these corrections did not materially alter the study’s main findings.
Fat Distribution Assessment
Fat distribution was assessed by measuring VFA and SFA via bioelectrical impedance analysis (BIA)21 using the DUALSCAN HDS-2000 instrument (Omron Healthcare, Kyoto, Japan). Measurements were taken after an overnight fast and voiding of the bladder. The device calculates body impedance through weak electrical currents passing through the body, and participant data (hospital number, height, weight, age, gender) were entered for precise calculations. The abdominal measurement unit on the device measured abdominal shape, and impedance measurements allowed for calculation of VFA and SFA according to the manufacturer’s protocols.
Statistical Analysis
Descriptive Statistics
The baseline characteristics of the study population are presented as means ± standard deviations for normally distributed continuous variables, medians (interquartile ranges) for skewed variables, and as frequencies and percentages for categorical variables. Differences in baseline characteristics were assessed using the t-test for normally distributed continuous variables, the Mann–Whitney U-test for skewed variables, and the Chi-square test for categorical variables. Skewed variables (eg, triglycerides [TG], alanine transaminase [ALT]) were analyzed without log transformation. The level of statistical significance was set at P<0.05.
Correlation Analysis
Correlations between levels of thyroid hormones (FT3, FT4, TSH) and fat distribution measures (VFA, SFA) were analyzed via Pearson or Spearman correlation analysis based on the distribution of the data. Correlation results were evaluated at a significant level of P<0.05.
Multivariate Regression Analysis
Multiple linear regression models were employed to evaluate the independent effects of FT3, FT4, and TSH on VFA and SFA after adjusting for potential confounding variables, including age, gender, and BMI. Based on multicollinearity analyses, BMI and WHR were excluded from final models involving FT4 to avoid obscuring its significance in relation to fat distribution. Regression coefficients, confidence intervals, and P-values were reported for all predictors. All analyses were conducted using a statistical software package with significance set at P<0.05.
Handling of Outliers
Extreme values identified for Cr, FT4, and TSH measurements were corrected based on statistical review and clinical evaluation to enhance the robustness of the findings. A total of five values were adjusted: two for Cr (151.72 and 103.9 μmol/L), one for FT4 (7.71 ng/dL), and two for TSH (97.743 mU/L and one additional unreported outlier). These corrections addressed data entry inconsistencies or biologically implausible values, thus improving data quality and minimizing potential bias. Sensitivity analyses performed to assess the impact of these corrections showed no material changes in the direction, magnitude, or statistical significance of the associations between thyroid hormone levels and fat distribution. All statistical analyses in this study were performed using SPSS version 28.0 (IBM Corp., Armonk, NY, USA) and R software (version 4.2.2, R Foundation for Statistical Computing, Vienna, Austria), with results considered statistically significant at P<0.05.
Results
Baseline Characteristics of the Study Population
The baseline characteristics of the study participants (n=2,956) are summarized in Table 1, with stratification by gender. The mean age of the participants was 56.2 years, and the study population included a higher proportion of males (58.6%) than females (41.4%). Males had a significantly higher WHR and systolic blood pressure than females (both P<0.001). Lifestyle factors also showed gender-specific differences, with males reporting higher smoking and alcohol consumption rates than females. BMI did not differ significantly between the male and female groups (P=0.077).
Table 1 The Basic Characteristics Stratified by Gender
Thyroid Hormone Levels and Fat Distribution in T2DM Patients
Table 2 presents the distribution of VFA and SFA across groups of patients with different categories of thyroid hormone levels (decreased, normal, and increased) for FT3, FT4, and TSH. Notably, patients with elevated FT3 levels (>3.8 pg/mL) show higher mean values for VFA (79.75 ± 40.12 cm²) and SFA (148.96 ± 57.06 cm²) compared to those with normal (1.8–3.8 pg/mL) or decreased (<1.8 pg/mL) FT3 levels (all P<0.001). Conversely, no significant variation in VFA and SFA values was observed for patients in different FT4 categories (decreased, normal, increased), suggesting a lesser impact of FT4 alone on fat distribution (P>0.05). Upon analysis according to the TSH categories, slightly higher values of VFA (82.39 ± 49.75 cm²) and SFA (162.44 ± 61.38 cm²) were observed in the elevated TSH group (>5.57 mU/L) compared with the decreased and normal TSH groups (all P<0.05 for both VFA and SFA).

Table 2 The Basic Characteristics Stratified by Age
Correlation Between Thyroid Hormone Levels and Fat Distribution in T2DM Patients
Correlation analyses revealed significant relationships between thyroid hormone levels and fat distribution parameters (Table 3). FT3 was positively correlated with both VFA (r=0.248, P<0.001) and SFA (r=0.190, P<0.001), indicating that a higher FT3 level is associated with greater fat accumulation. FT4, on the other hand, tended to show a weak inverse correlation with both VFA (r=–0.018, P=0.321) and SFA (r=–0.019, P=0.283), although these relationships were not statistically significant. TSH showed a weak but significant positive correlation with both VFA (r=0.064, P=0.001) and SFA (r=0.072, P<0.001), indicating an association with fat distribution but a weaker association than that observed between FT3 and fat distribution.

Table 3 The Distribution of VFA and SFA According to Thyroid Hormones
Correlation Between Thyroid Hormone Levels and Fat Distribution
Correlation analyses revealed significant associations between thyroid hormone levels and fat distribution parameters (Table 4). FT3 was positively correlated with both VFA (r = 0.248, P < 0.001) and SFA (r = 0.190, P < 0.001), indicating that higher FT3 levels were associated with greater visceral and subcutaneous fat accumulation. FT4 showed weak negative correlations with VFA (r = –0.018, P = 0.321) and SFA (r = –0.019, P = 0.283), but these associations were not statistically significant. TSH demonstrated weak yet significant positive correlations with both VFA (r = 0.064, P = 0.001) and SFA (r = 0.072, P < 0.001), although the strength of these relationships was less pronounced compared with FT3.

Table 4 The Correlation Between Thyroid Hormones and VFA and SFA
Predictive Values of Thyroid Hormones for Abnormal Fat Distribution
To further evaluate the independent effects of thyroid hormones on fat distribution, multivariate linear regression models were constructed after adjusting for potential confounding factors, including age, gender, and BMI (Table 5). FT3 remained independently and positively associated with both VFA (Coef = 8.06, 95% CI: 5.87–10.24, P < 0.001) and SFA (Coef = 9.71, 95% CI: 6.62–12.79, P < 0.001). In contrast, FT4 was inversely associated with VFA (Coef = –12.20, 95% CI: –17.36 to –7.03, P < 0.001) and SFA (Coef = –16.68, 95% CI: –23.98 to –9.38, P < 0.001), suggesting a potential protective role of FT4 against abnormal fat accumulation. TSH did not show significant associations with either VFA or SFA (all P > 0.05), indicating a relatively minor impact compared with FT3 and FT4.

Table 5 The Multivariate Linear Regression Between Thyroid Hormones and VFA and SFA
Gender and Age-Specific Variations in Fat Distribution Among T2DM Patients
Additional analyses with patient stratification by age and gender were conducted to further elucidate the relationships between thyroid hormone levels and fat distribution in T2DM patients. As shown in Table S1, the associations between FT3 and fat distribution parameters were consistent across age groups, with younger and middle-aged adults showing similar patterns of correlation for FT3 with VFA and SFA. Among women, FT4 exhibited a more pronounced inverse relationship with VFA, and this effect was not observed in men. Gender differences were also evident in the correlation of TSH and fat distribution, with men showing a stronger association of TSH with VFA and SFA compared with women (P<0.05 for interaction).
Discussion
The present investigation of the associations between thyroid hormone levels (FT3, FT4, and TSH) and fat distribution, specifically VFA and SFA, in T2DM patients revealed the following significant associations: FT3 was positively correlated with both VFA and SFA, while FT4 was inversely associated with these fat distribution measures. TSH displayed a weak positive association that did not persist after adjustment for confounding variables. These results suggest a nuanced role for thyroid hormones in fat metabolism and distribution in T2DM patients with normal thyroid function, supporting a potential role for FT3 and FT4 as biomarkers for evaluating metabolic risk in this population.
The strong positive associations between FT3 and both VFA and SFA in our cohort align with FT3’s known role as an active thyroid hormone involved in regulating metabolic rate and energy expenditure.22 FT3’s influence on lipolysis and fat accumulation may explain the observed associations, as higher FT3 levels likely stimulate greater metabolic turnover, promoting fat mobilization and deposition, particularly in visceral regions.23 The accumulation of visceral fat is closely linked to insulin resistance, systemic inflammation, and cardiovascular complications, all of which are prevalent in T2DM patients.24 Our findings are consistent with those of prior studies25,26 indicating that elevated FT3 levels are associated with higher body fat percentages and greater metabolic risks, especially in populations predisposed to obesity and diabetes.
The relationship between FT3 and fat distribution also could be influenced by the unique metabolic environment of T2DM.27 Insulin resistance, a hallmark of T2DM, is associated with altered lipid metabolism and may exacerbate FT3’s effects on fat accumulation.28 While FT3 promotes lipid oxidation and energy consumption, its effects in the context of T2DM may be compounded by impaired glucose and lipid regulation, resulting in disproportionate fat deposition, particularly in visceral areas.29 Thus, T2DM patients with elevated FT3 levels may be at increased risk of accumulating metabolically active visceral fat, predisposing them to worsened metabolic control and cardiovascular risk. This highlights the potential utility of FT3 as a biomarker for assessing fat-related metabolic risks in T2DM.30
The observed positive association between FT3 levels and fat accumulation may appear paradoxical considering the known lipolytic effects of FT3. However, previous research has suggested several mechanisms underlying this relationship in insulin-resistant states. Elevated FT3 levels in T2DM patients could reflect compensatory hypersecretion intended to overcome reduced thyroid hormone sensitivity at peripheral tissues,30 potentially due to alterations in thyroid hormone receptor expression or signaling pathways. Additionally, impaired peripheral conversion of T4 to active T3 caused by chronic inflammation or metabolic dysfunction may lead to higher FT3 secretion from the thyroid gland to maintain metabolic homeostasis. These compensatory adjustments may paradoxically leads to the association of elevated circulating FT3 levels with greater fat accumulation, especially visceral fat, in individuals with insulin resistance and metabolic disturbances.31
Unlike FT3, FT4 displayed an inverse relationship with VFA and SFA, suggesting a potential protective role of FT4 in fat metabolism among T2DM patients.32 FT4, often considered less metabolically active than FT3, appears to exert a regulatory influence on fat storage, potentially attenuating fat accumulation in both visceral and subcutaneous regions.33 This finding aligns with the hypothesis that FT4 supports metabolic homeostasis and thereby contributes to favorable fat distribution patterns in T2DM patients. Previous studies14,34 have shown that lower FT4 levels are linked to increased fat mass and metabolic risk, further corroborating our finding that higher FT4 levels might protect against excessive fat accumulation.
Our gender-stratified analysis suggested that the protective effect of FT4 may be more pronounced in females than in males. A potential mechanism may be related to estrogen. Estrogen has been shown to influence fat distribution by favoring subcutaneous over visceral fat storage, and this effect may interact with the influence of FT4 on metabolism.35 In postmenopausal women, who experience shifts in both thyroid hormone levels and fat distribution, maintaining adequate FT4 levels might help mitigate visceral fat accumulation.36 This interplay warrants further investigation to explore whether FT4 could be targeted in gender-specific interventions aimed at improving fat distribution and metabolic outcomes in T2DM patients.
TSH demonstrated a weak positive correlation with VFA and SFA, which did not remain significant after adjustment for age, gender, and BMI.37 As a regulatory hormone, TSH primarily influences thyroid hormone synthesis and secretion rather than directly impacting metabolism or fat storage. This may explain its limited association with fat distribution in our cohort. Although previous research38 suggested a link between higher TSH levels and obesity, our results indicate that TSH may have minimal impact on fat distribution in euthyroid individuals with normal FT3 and FT4 levels. The lack of a significant association between TSH and fat accumulation after adjustment highlights the importance of considering FT3 and FT4 independently when assessing thyroid hormone-related metabolic risks in T2DM patients.
The relatively weak and statistically nonsignificant adjusted associations observed between TSH and fat distribution parameters may be explained by the role of TSH primarily as a centrally controlled regulator of thyroid function, rather than a direct peripheral mediator of metabolic processes. TSH exerts indirect metabolic effects by influencing thyroid hormone synthesis and secretion, whereas peripheral tissues primarily respond to circulating FT3 and FT4 levels. Furthermore, existing research supports the notion that peripheral metabolic activities and fat metabolism are predominantly regulated by thyroid hormones at the tissue level, with TSH having limited direct effects outside the central axis.39 These findings align with our observation that FT3 and FT4 showed more robust associations with fat distribution compared with TSH.
Overall, our results are consistent with previous studies40,41 that described a positive association of FT3 with body fat and an inverse relationship of FT4 with fat accumulation. Similar findings in general populations suggest that these thyroid hormone relationships extend to T2DM patients, who exhibit distinct metabolic profiles due to insulin resistance and dyslipidemia. Studies in non-diabetic populations have linked higher FT3 levels with increased visceral and subcutaneous fat, reinforcing our observations that FT3 may predispose T2DM patients to adverse fat accumulation patterns.42 Moreover, a study by Mele et al36 demonstrated that FT4 levels are inversely correlated with adiposity, suggesting that FT4 might contribute to a healthier fat distribution pattern. However, our study’s focus on euthyroid T2DM patients provides novel insights and fills a gap in the literature, as few studies have exclusively examined this population.
As noted above, the significant associations between FT3 and measures of fat distribution suggest that FT3 could serve as a useful biomarker for identifying T2DM patients at risk for excessive visceral fat accumulation. Accordingly, monitoring of FT3 levels in T2DM patients may help clinicians identify individuals with heightened metabolic and cardiovascular risks linked to visceral adiposity. Moreover, our finding regarding the protective role of FT4 against abnormal fat distribution highlight the potential benefits of preserving or optimizing FT4 levels to promote a favorable fat distribution in T2DM patients. Personalized treatment approaches that consider individual thyroid hormone profiles could enhance metabolic management, potentially facilitating measures to reduce fat accumulation and improve metabolic outcomes in T2DM patients. Interventions focusing on lifestyle modification or pharmacotherapy tailored to regulate patients’ FT3 and FT4 levels could provide targeted benefits.
The observed gender-specific differences in the effect of FT4 on fat distribution underscore the importance of considering gender in metabolic research. Among the women in our study, we observed a more pronounced inverse relationship between FT4 and VFA, suggesting that FT4 may offer stronger protective effects in women. Estrogen likely interacts with FT4, influencing fat distribution patterns that favour subcutaneous over visceral storage. This interaction is particularly relevant for postmenopausal women, who experience hormonal shifts that might exacerbate visceral fat accumulation. Thus, strategies to maintain adequate FT4 levels could mitigate these effects, supporting metabolic health in postmenopausal T2DM patients. Future studies should explore the existence and effects of interactions between estrogen and thyroid hormones to better understand their combined effects on fat distribution in T2DM patients.
The present study also has several limitations that should be considered. The cross-sectional design prevents us from establishing causality, leaving it unclear whether thyroid hormone changes precede or result from altered fat distribution. A longitudinal study design is required to clarify causal pathways. Additionally, the exclusion of T2DM patients with thyroid dysfunction restricts the generalizability of our findings, as the relationship between thyroid hormones and fat distribution may differ in patients with hypo- or hyperthyroidism. The used of BIA for measurement of VFA and SFA may also limit the precision of the data, compared with that produced by imaging modalities such as MRI or CT, but BIA offers the advantage of being a practical, non-invasive method for large-scale studies. Residual confounding is another limitation. Although we controlled age, gender, BMI, education, blood pressure, and lipid profiles, other clinically relevant factors were not available in our dataset. Examples include the duration of diabetes, glycemic control (HbA1c), the use of specific antidiabetic medications, smoking status, and physical activity. These factors may influence both thyroid hormone metabolism and fat distribution, and their absence from our analysis may introduce bias. Therefore, residual confounding cannot be excluded and should be considered when interpreting our findings. Future studies should aim to account for these variables to provide a more comprehensive understanding of the impacts of different thyroid hormones on fat distribution.
Finally, this study provides insight for the directions of future research. Longitudinal investigations tracking thyroid hormone levels and fat distribution over time are needed to clarify potential causal relationships in T2DM populations. Complementary mechanistic studies, including observational analyses and experimental models, may help determine whether variations in FT3 and FT4 drive fat accumulation or represent compensatory responses. Elucidating the molecular pathways linking thyroid hormones with lipid metabolism will be particularly important. In addition, examination of other thyroid hormone metabolites, such as T2 and reverse triiodothyronine (rT3), may broaden our understanding of thyroid hormone regulation of fat distribution and metabolic health.
Conclusion
In conclusion, this study highlights significant associations between thyroid hormones and fat distribution in euthyroid T2DM patients. Elevated FT3 levels were linked to increased visceral and subcutaneous fat, whereas higher FT4 levels were associated with more favourable fat distribution. These findings provide new insights into the metabolic roles of thyroid hormones and suggest that they may serve as useful indicators for identifying patients at higher metabolic risk. Future longitudinal and mechanistic studies are warranted to validate these associations and to explore their potential relevance for personalized metabolic management in T2DM.
Data Sharing Statement
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study was approved by the Institutional Review Board of the Affiliated Hospital of Southwest Medical University. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all participants prior to enrolment.
Acknowledgments
We thank Medjaden, Inc. for scientific editing of this paper.
Funding
The study was funded by the Youth Fund of Medical Association (No. 16213).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hurtado MD, Vella A. What is type 2 diabetes? Medicine. 2019;47(1):10–15. doi:10.1016/j.mpmed.2018.10.010
2. Abdul Basith Khan M, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes — global burden of disease and forecasted trends. J Epidemiol Global Health. 2020;10(1):107–111. doi:10.2991/jegh.k.191028.001
3. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
4. Zakir M, Ahuja N, Surksha MA, et al. Cardiovascular complications of diabetes: from microvascular to macrovascular pathways. Cureus. 2023;15(9):e45835. doi:10.7759/cureus.45835
5. Chandrasekaran P, Weiskirchen R. The Role of Obesity in Type 2 Diabetes Mellitus—An Overview. Int J Mol Sci. 2024;25(3):1882. doi:10.3390/ijms25031882
6. Goswami B, Reang T, Sarkar S, Sengupta S, Bhattacharjee B. Role of body visceral fat in hypertension and dyslipidemia among the diabetic and nondiabetic ethnic population of Tripura—A comparative study. J Family Med Primary Care. 2020;9(6).
7. Ruze R, Liu T, Zou X, et al. Obesity and type 2 diabetes mellitus: connections in epidemiology, pathogenesis, and treatments. Front Endocrinol. 2023;14.
8. Yavuz S, Salgado Nunez Del Prado S, Celi FS. Thyroid hormone action and energy expenditure. J Endocrine Soc. 2019;3(7):1345–1356. doi:10.1210/js.2018-00423
9. Wang X, Wu Z, Liu Y, et al. The role of thyroid-stimulating hormone in regulating lipid metabolism: implications for body–brain communication. Neurobiol Dis. 2024;201:106658. doi:10.1016/j.nbd.2024.106658
10. Bansal S, Vachher M, Arora T, Kumar B, Burman A. Visceral fat: a key mediator of NAFLD development and progression. Hum Nutr Metab. 2023;33:200210. doi:10.1016/j.hnm.2023.200210
11. Sterenborg RBTM, Steinbrenner I, Li Y, et al. Multi-trait analysis characterizes the genetics of thyroid function and identifies causal associations with clinical implications. Nat Commun. 2024;15(1):888. doi:10.1038/s41467-024-44701-9
12. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–1751. doi:10.1089/thy.2014.0028
13. Cappola AR, Arnold AM, Wulczyn K, Carlson M, Robbins J, Psaty BM. Thyroid function in the euthyroid range and adverse outcomes in older adults. J Clin Endocrinol Metab. 2015;100(3):1088–1096. doi:10.1210/jc.2014-3586
14. Gokkaya N, Aydin K. Efficacy of levothyroxine monotherapy in achieving clinical euthyroidism and its impact on weight loss in women with hypothyroidism and obesity. Scientific Reports. 2024;14(1):27822. doi:10.1038/s41598-024-78185-w
15. Kim JM, Kim BH, Lee H, et al. The relationship between thyroid function and different obesity phenotypes in Korean euthyroid adults. Diabetes Metab J. 2019;43(6):867–878. doi:10.4093/dmj.2018.0130
16. Teixeira PFS, Dos Santos PB, Pazos-Moura CC. The role of thyroid hormone in metabolism and metabolic syndrome. Therapeu Adv Endocrinol Metabo. 2020;11:2042018820917869. doi:10.1177/2042018820917869
17. Abiri B, Ahmadi AR, Mahdavi M, Amouzegar A, Valizadeh M. Association between thyroid function and obesity phenotypes in healthy euthyroid individuals: an investigation based on Tehran Thyroid Study. Eur J Med Res. 2023;28(1):179. doi:10.1186/s40001-023-01135-1
18. Adamska A, Raczkowski A, Stachurska Z, et al. Body composition and serum concentration of thyroid hormones in euthyroid men and women from general population. J Clin Med. 2022;11(8):2118. doi:10.3390/jcm11082118
19. Hatziagelaki E, Paschou SA, Schön M, Psaltopoulou T, Roden M. NAFLD and thyroid function: pathophysiological and therapeutic considerations. Trends Endocrinol Metab. 2022;33(11):755–768. doi:10.1016/j.tem.2022.08.001
20. Ward CR. Chapter 69 – Thyroid Storm. In: Silverstein DC, Hopper K, editors. Small Animal Critical Care Medicine.
2nd ed. St. Louis: W.B. Saunders; 2015:364–367.21. Lai C-L, Lu H-K, Huang A-C, Chu L-P, Chuang H-Y, Hsieh K-C. Bioimpedance analysis combined with sagittal abdominal diameter for abdominal subcutaneous fat measurement. Front Nutr. 2022;9.
22. Hoermann R, Pekker MJ, Midgley JEM, Dietrich JW. The role of supporting and disruptive mechanisms of FT3 homeostasis in regulating the hypothalamic–pituitary–thyroid axis. Therapeu Adv Endocrinol Metabo. 2023;14:20420188231158163. doi:10.1177/20420188231158163
23. Hu Y, Zhou F, Lei F, et al. The nonlinear relationship between thyroid function parameters and metabolic dysfunction-associated fatty liver disease. Front Endocrinol. 2023;14.
24. Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55(1):31–55. doi:10.1016/j.immuni.2021.12.013
25. Sun X, Chen L, Wu R, Zhang D, He Y. Association of thyroid hormone with body fat content and lipid metabolism in euthyroid male patients with type 2 diabetes mellitus: a cross-sectional study. BMC Endocr Disord. 2021;21(1):241. doi:10.1186/s12902-021-00903-6
26. Yu L, Liu Y, Wang Y, et al. Increased thyroid hormone sensitivity is correlated with visceral obesity in patients with type 2 diabetes. Lipids health Dis. 2024;23(1):337. doi:10.1186/s12944-024-02320-9
27. Bouazza A, Favier R, Fontaine E, Leverve X, Koceir E-A. Potential applications of thyroid hormone derivatives in obesity and type 2 diabetes: focus on 3,5-diiodothyronine (3,5-T2) in psammomys obesus (fat sand rat) model. Nutrients. 2022;14(15):3044. doi:10.3390/nu14153044
28. DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53(7):1270–1287. doi:10.1007/s00125-010-1684-1
29. Emiroğlu C, Özdemir Ç, Görpelioğlu S, Aypak C. The relationship between thyroid hormones, metabolic syndrome and HOMA-IR in people with obesity or overweight. Clin Diabetol. 2022;11(5):333–339. doi:10.5603/DK.a2022.0043
30. Biondi B, Kahaly GJ, Robertson RP. Thyroid dysfunction and diabetes mellitus: two closely associated disorders. Endocrin Rev. 2019;40(3):789–824. doi:10.1210/er.2018-00163
31. Holtorf K. Peripheral thyroid hormone conversion and its impact on TSH and metabolic activity. J Restorat Med. 2014;3(1):30–52. doi:10.14200/jrm.2014.3.0103
32. Cao B, Li K, Ke J, Zhao D. Impaired sensitivity to thyroid hormones is associated with the change of abdominal fat in euthyroid type 2 diabetes patients: a retrospective cohort study. J Diabe Res. 2024;2024(1):8462987. doi:10.1155/2024/8462987
33. Freedland ES. Role of a critical visceral adipose tissue threshold (CVATT) in metabolic syndrome: implications for controlling dietary carbohydrates: a review. Nutr Metab. 2004;1(1):12.
34. Sanyal D, Raychaudhuri M. Hypothyroidism and obesity: an intriguing link. Indian J Endocrinol Metab. 2016;20(4):554–557. doi:10.4103/2230-8210.183454
35. Mazza E, Troiano E, Ferro Y, et al. Obesity, dietary patterns, and hormonal balance modulation: gender-specific impacts. Nutrients. 2024;16(11):1629. doi:10.3390/nu16111629
36. Mele C, Mai S, Cena T, et al. The pattern of TSH and fT4 levels across different BMI ranges in a large cohort of euthyroid patients with obesity. Front Endocrinol. 2022;13.
37. Shaoba A, Basu S, Mantis S, Minutti C. Serum thyroid-stimulating hormone levels and body mass index percentiles in children with primary hypothyroidism on levothyroxine replacement. J Clin Res Pediatr Endocrinol. 2017;9(4):337–343. doi:10.4274/jcrpe.3661
38. Witte T, Völzke H, Lerch MM, et al. Association between serum thyroid-stimulating hormone levels and visceral adipose tissue: a population-based study in Northeast Germany. Euro Thyroid J. 2017;6(1):12–19. doi:10.1159/000450977
39. McAninch EA, Bianco AC. Thyroid hormone signaling in energy homeostasis and energy metabolism. Ann N Y Acad Sci. 2014;1311:77–87. doi:10.1111/nyas.12374
40. Biondi B. Thyroid and obesity: an intriguing relationship. J Clin Endocrinol Metab. 2010;95(8):3614–3617. doi:10.1210/jc.2010-1245
41. Mullur R, Liu -Y-Y, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355–382. doi:10.1152/physrev.00030.2013
42. Tacke F, Horn P, Wai-Sun Wong V, et al. EASL–EASD–EASO clinical practice guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81(3):492–542.
Continue Reading
