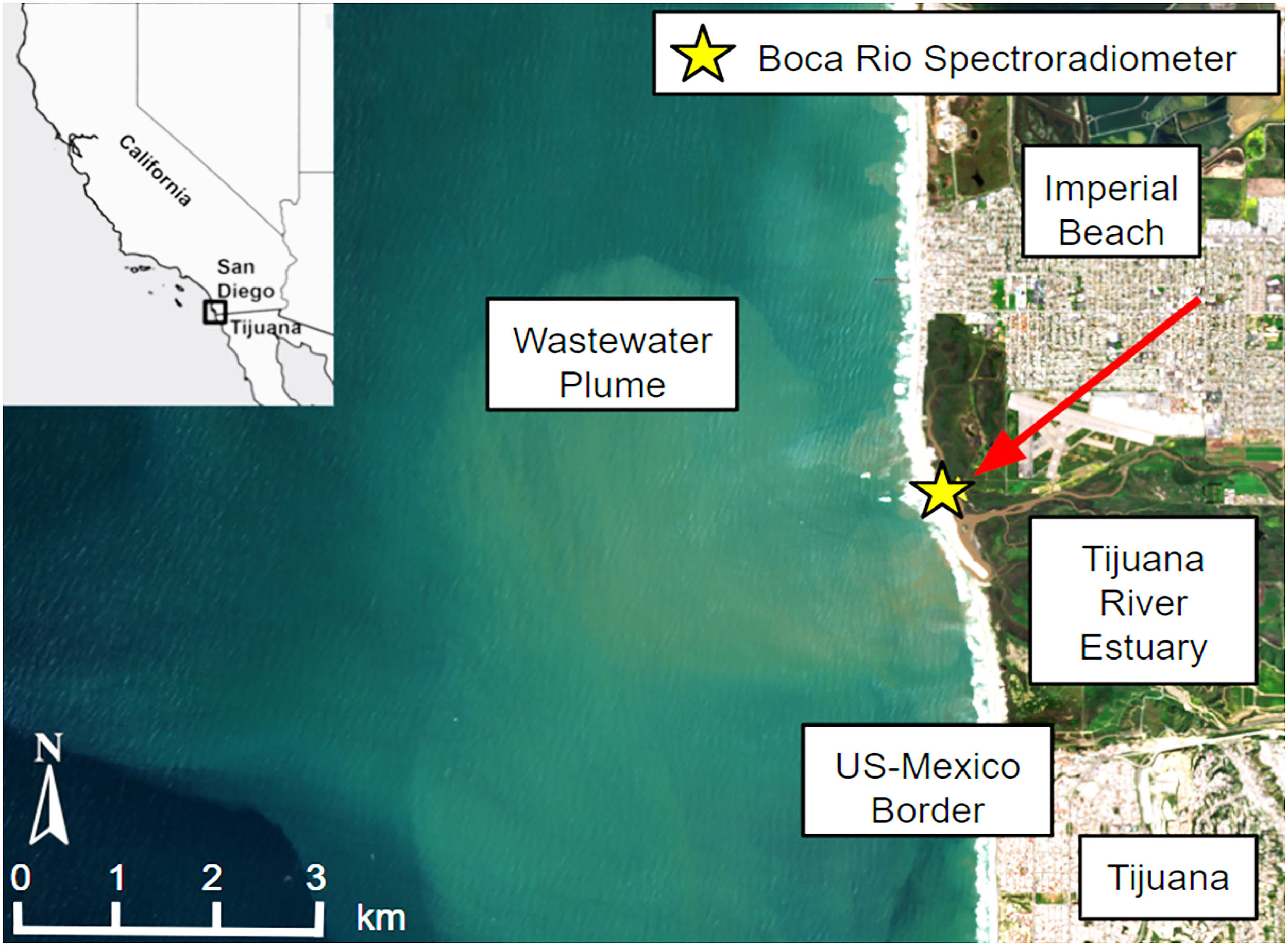
NASA’s imaging spectrometer riding on the International Space Station has spotted a wastewater plume pouring from the Tijuana River into the Pacific. The signal is strong enough that scientists can track the pollution from orbit with a single…
Blog
-
mPFS 11.14 Months (HR=0.6, P
- Ivonescimab plus chemotherapy demonstrated a median PFS of 11.14 months, PFS HR=0.60, P < 0.0001.
- The absolute difference in median PFS between the two groups was 4.24 months (ΔPFS = 4.24 months), indicating significantly prolonged progression-free survival with ivonescimab combination therapy.
- Significant benefits were consistently observed with ivonescimab plus chemotherapy versus tislelizumab combination irrespective of PD-L1 expression.
- Ivonescimab combination therapy showed significant benefit over tislelizumab regimen in patients with or without liver metastases and with or without brain metastases.
- In the HARMONi-6 study, 92.3% of enrolled patients had stage IV disease, and approximately 63% had central-type squamous cell carcinoma.
- Ivonescimab demonstrated a favorable overall safety profile with no new safety signals identified.
- Overall survival (OS) data were not yet mature at the time of this analysis.
HONG KONG, Oct. 19, 2025 /PRNewswire/ — Akeso (9926.HK) announced the groundbreaking results of the registrational Phase III clinical study (AK112-306/HARMONi-6) evaluating ivonescimab (PD-1/VEGF bispecific antibody), a globally first-in-class bispecific antibody developed by Akeso, in combination with chemotherapy versus tislelizumab combined with chemotherapy as first-line treatment for advanced squamous non-small cell lung cancer (sq-NSCLC). The study results were presented by Professor Lu Shun, Chief of the Shanghai Lung Cancer Center at Shanghai Chest Hospital and Professor of Medicine at Shanghai Jiaotong University, at the 2025 ESMO Presidential Symposium, and the results of the HARMONi-6 clinical trial were simultaneously published in The Lancet.
The HARMONi-6 study met its primary endpoint of progression-free survival (PFS), demonstrating a decisive and strong positive outcome with both statistically significant and clinically meaningful benefits. Ivonescimab plus chemotherapy substantially prolonged sq-NSCLC patient’s progression-free survival compared to tislelizumab plus chemotherapy:
- The progression-free survival (PFS) hazard ratio (HR) was 0.60 (p < 0.0001) for ivonescimab plus chemotherapy versus tislelizumab plus chemotherapy. The median PFS was 11.14 months in the ivonescimab combination group versus 6.90 months in the tislelizumab combination group, representing an absolute improvement of ΔPFS = 4.24 months.
- Consistent clinical benefits were observed across all PD-L1 expression subgroups. In the PD-L1 negative (TPS <1%) population, the PFS HR was 0.55 for ivonescimab plus chemotherapy versus tislelizumab plus chemotherapy. In the PD-L1 positive (TPS ≥1%) population, the PFS HR was 0.66 for ivonescimab plus chemotherapy versus tislelizumab plus chemotherapy.
- Regardless of liver or brain metastasis, ivonescimab combined with chemotherapy showed significant benefit over the tislelizumab-based regimen. Among patients with liver metastases, the PFS HR was 0.53; among those without liver metastases, the PFS HR was 0.64. For patients with ≥3 baseline metastatic sites, the PFS HR was 0.46, and for those with <3 baseline metastatic sites, the PFS HR was 0.64.
The ivonescimab group demonstrated a favorable overall safety profile with no new safety signals identified. The incidence of treatment-related serious adverse reactions and grade 3 or higher bleeding events was comparable to the tislelizumab regimen group. The most common adverse reactions were chemotherapy-associated myelosuppression.
The HARMONi-6 study enrolled 532 subjects, with balanced baseline characteristics between the two groups. 92.3% of subjects had clinical stage IV disease. The squamous carcinoma characteristics of enrolled patients reflected real-world clinical presentations, with central-type squamous carcinoma accounting for approximately 63% of the total patients (66.9% in the ivonescimab group vs. 59.4% in the tislelizumab group), consistent with real-world patient distribution. PD-L1 expression levels also reflected those seen in real-world clinical settings.
Approved in 2024, ivonescimab has demonstrated groundbreaking clinical value across dozens of clinical studies and real-world treatments involving over 40,000 patients. In the field of tumor immunotherapy, whether compared with PD-1 monotherapy or PD-1 combination chemotherapy (the current standard of care for many cancers), as well as in the field of anti-angiogenesis therapy compared to VEGF-targeted regimens, ivonescimab has demonstrated robust and superior clinical efficacy, highlighting its exceptional capacity to drive iterative advances in cancer treatment.
The encouraging results from the HARMONi-6 study have led to the review of a supplemental New Drug Application (sNDA) in China for ivonescimab in combination with chemotherapy as a first-line treatment for advanced squamous NSCLC. Meanwhile, the global enrollment for the international, multicenter Phase III HARMONi-3 trial, assessing ivonescimab as a first-line treatment for both squamous (sq-NSCLC) and non-squamous (nsq-NSCLC) non-small cell lung cancer is ongoing.
Forward-Looking Statement of Akeso, Inc.
This announcement by Akeso, Inc. (9926.HK, “Akeso”) contains “forward-looking statements”. These statements reflect the current beliefs and expectations of Akeso’s management and are subject to significant risks and uncertainties. These statements are not intended to form the basis of any investment decision or any decision to purchase securities of Akeso. There can be no assurance that the drug candidate(s) indicated in this announcement or Akeso’s other pipeline candidates will obtain the required regulatory approvals or achieve commercial success. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements.Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of pharmaceutical industry regulation and health care legislation in P.R.China, the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; Akeso’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of the Akeso’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions.
Akeso does not undertake any obligation to publicly revise these forward-looking statements to reflect events or circumstances after the date hereof, except as required by law.
About Akeso
Akeso (HKEX: 9926.HK) is a leading biopharmaceutical company committed to the research, development, manufacturing and commercialization of the world’s first or best-in-class innovative biological medicines. Founded in 2012, the company has created a unique integrated R&D innovation system with the comprehensive end-to-end drug development platform (ACE Platform) and bi-specific antibody drug development technology (Tetrabody) as the core, a GMP-compliant manufacturing system and a commercialization system with an advanced operation mode, and has gradually developed into a globally competitive biopharmaceutical company focused on innovative solutions. With fully integrated multi-functional platform, Akeso is internally working on a robust pipeline of over 50 innovative assets in the fields of cancer, autoimmune disease, inflammation, metabolic disease and other major diseases. Among them, 24 candidates have entered clinical trials (including 15 bispecific/multispecific antibodies and bispecific ADCs. Additionally, 7 new drugs are commercially available. Through efficient and breakthrough R&D innovation, Akeso always integrates superior global resources, develops the first-in-class and best-in-class new drugs, provides affordable therapeutic antibodies for patients worldwide, and continuously creates more commercial and social values to become a global leading biopharmaceutical enterprise.For more information, please visit https://www.akesobio.com/en/about-us/corporate-profile/ and follow us on Linkedin.
SOURCE Akeso, Inc.

Continue Reading
-

Former Big Brother contestant sues for disability after washing dishes on set
Former Big Brother contestant Shilo Shalom has filed a lawsuit against the National Insurance Institute, demanding recognition of what he claims is a lasting disability stemming from an injury he sustained while washing dishes during the 2024…
Continue Reading
-

‘Battlefield 6’ Season 1 release date, content, updates, and more
Battlefield 6 has had a stellar first week, becoming the most successful game in the franchise and EA has no plans to stop supporting the game as new content is planned before the end of the month.
- READ MORE: ‘Battlefield 6’ review: modern…
Continue Reading
-

Abadia Fall 2025 at Riyadh Fashion Week – WWD
- Abadia Fall 2025 at Riyadh Fashion Week WWD
- Vivienne Westwood Show Opens Riyadh Fashion Week, as Saudis Highlight Creative Side The New York Times
- Saudi labels ‘honor roots’ at Riyadh Fashion Week Arab News
- Mushroom skin bag and Riyadh…
Continue Reading
-

Smart jab can shrink head and neck cancer tumours within six weeks, trial finds | Cancer research
Doctors have hailed “incredibly encouraging” trial results that show a triple-action smart jab can shrink tumours in head and neck cancer patients within six weeks.
Head and neck cancer is the world’s sixth most common form of the disease. If it spreads or comes back after standard treatment, patients may be offered immunotherapy and platinum chemotherapy. But if this fails, there is often little else doctors can do.
Research showed a drug called amivantamab, given as an injection, can shrink tumours in patients with recurrent or metastatic cancer who had tried immunotherapy and chemotherapy. Details were presented at the European Society for Medical Oncology conference in Berlin.
Prof Kevin Harrington, a professor of biological cancer therapies at the Institute of Cancer Research in London, and consultant oncologist at the Royal Marsden NHS foundation trust, said: “To see this level of benefit for patients who have endured numerous treatments is incredibly encouraging.
“This could represent a real shift in how we treat head and neck cancer – not just in terms of effectiveness, but also in how we deliver care.”
He added: “This is the first time we’ve tested this kind of triple-action therapy for head and neck cancer patients whose disease has returned after treatment. Amivantamab is a smart drug that not only blocks two key cancer pathways but also helps the immune system do its job.
“Unlike many cancer treatments that require hours in a hospital chair, amivantamab is given as a simple injection under the skin. This makes it faster, more convenient, and potentially easier to deliver in outpatient clinics – or even at home in the future.”
The Orig-AMI 4 trial, funded by the pharmaceuticals company Janssen, involved patients from 11 countries, including the UK. Each had recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) – a hard-to-treat form that often returns after standard therapies.
One group of 86 patients in the study, who had received immunotherapy and chemotherapy, were given amivantamab. Early results show 76% of this group saw their tumours shrink or stop growing.
Responses were seen within six weeks on average and treatment was generally well tolerated. Most side-effects were mild to moderate. Average progression-free survival of patients receiving amivantamab on its own was 6.8 months.
Amivantamab is a drug that targets cancer in three ways. It blocks both EGFR (epidermal growth factor receptor), a protein that helps tumours grow, and MET, a pathway that cancer cells often use to escape treatment. It also helps activate the immune system to attack the tumour.
Carl Walsh has tongue cancer and joined the trial in July after chemotherapy and immunotherapy failed. “I’m now on my seventh cycle of treatment. It’s working well so far and I’m very happy with the progress,” the 59-year-old from Birmingham said.
“Before starting the trial, I couldn’t talk properly and eating was difficult but the swelling has gone down a lot, and I’m not in the same amount of pain I used to be in. Sometimes I even forget that I have cancer.”
Continue Reading
-

KP governor calls on Federal Interior Minister, discuss security situation in KP
LAHORE: Governor Khyber Pakhtunkhwa Faisal Karim Kundi met with Federal Interior Minister Mohsin Naqvi at his residence in Lahore on Sunday to discuss the overall law and order situation in the province and recent incidents of terrorism.
During…
Continue Reading
-
![Leem Fall 2025 at Riyadh Fashion Week [PHOTOS] – WWD](https://afnnews.qaasid.com/wp-content/uploads/2025/10/leem-f25-riyadh-08.jpg)
Leem Fall 2025 at Riyadh Fashion Week [PHOTOS] – WWD
- Leem Fall 2025 at Riyadh Fashion Week [PHOTOS] WWD
- Vivienne Westwood Show Opens Riyadh Fashion Week, as Saudis Highlight Creative Side The New York Times
- Saudi labels ‘honor roots’ at Riyadh Fashion Week Arab News
- Mushroom skin bag and Riyadh…
Continue Reading
-

Police looking into claims Prince Andrew asked officer to find information on Virginia Giuffre | Prince Andrew
The Metropolitan police are looking into claims that Prince Andrew asked his taxpayer-funded close protection officer to uncover information about Virginia Giuffre hours before the emergence of a bombshell picture of them together.
Leaked emails…
Continue Reading