Sucker Punch has released a new patch for Ghost of Yōtei with numerous bug and crash fixes, including one that could rarely occur when using ray tracing.
Those who purchased the Digital Deluxe Edition but didn’t receive the Black Ghost Mask…

Sucker Punch has released a new patch for Ghost of Yōtei with numerous bug and crash fixes, including one that could rarely occur when using ray tracing.
Those who purchased the Digital Deluxe Edition but didn’t receive the Black Ghost Mask…

Adding apalutamide (Erleada) to androgen deprivation therapy (ADT) in patients with biochemically recurrent prostate cancer reduced the risk of developing metastases and castration-resistant disease, according to findings from the phase 3 PRESTO…
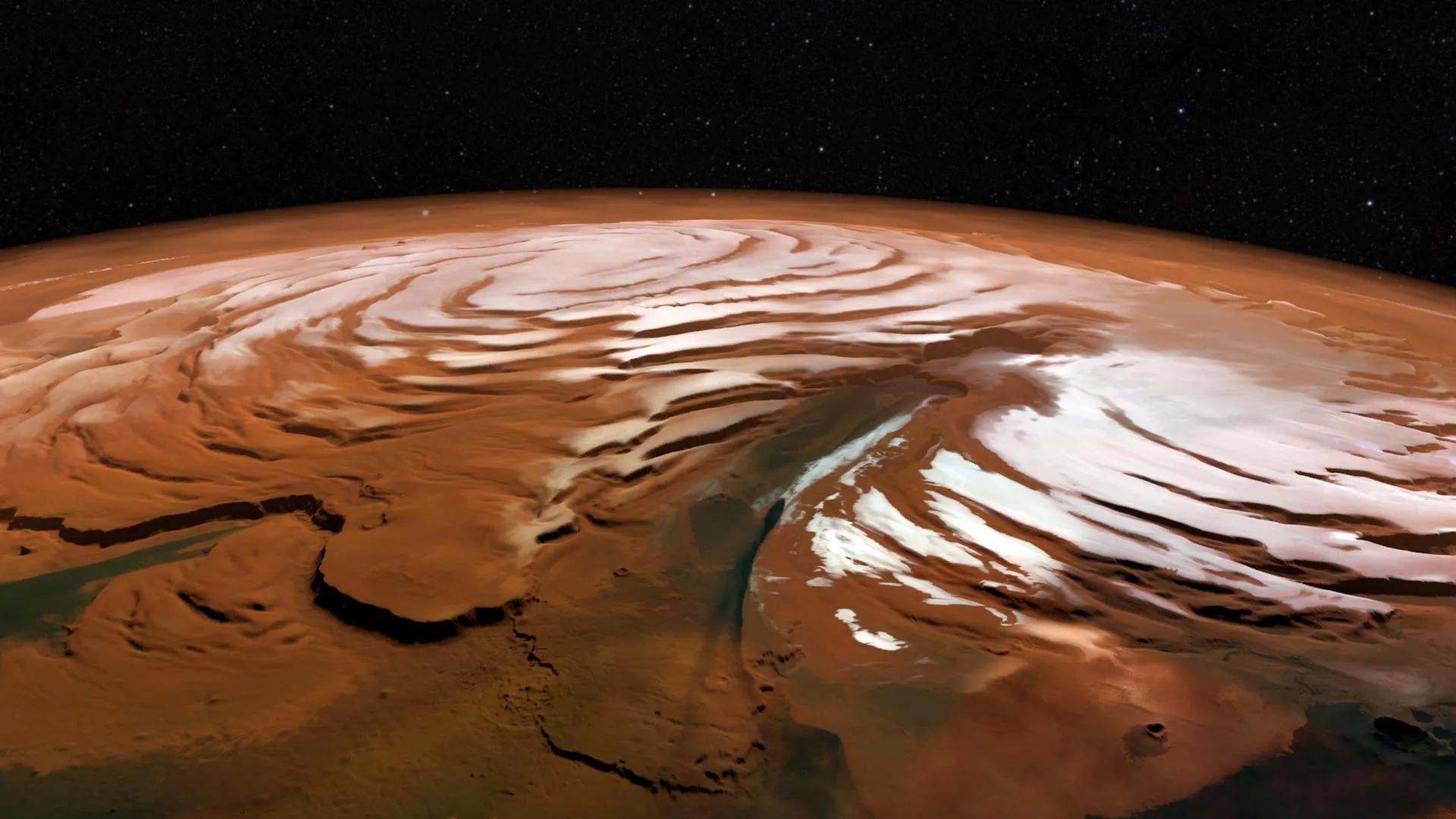
Scientists have recently captured a rare look at the harsh winter conditions swirling above Mars’ north pole. Inside the planet’s polar vortex, temperatures drop dramatically — much colder than the air outside — and the continuous darkness of…
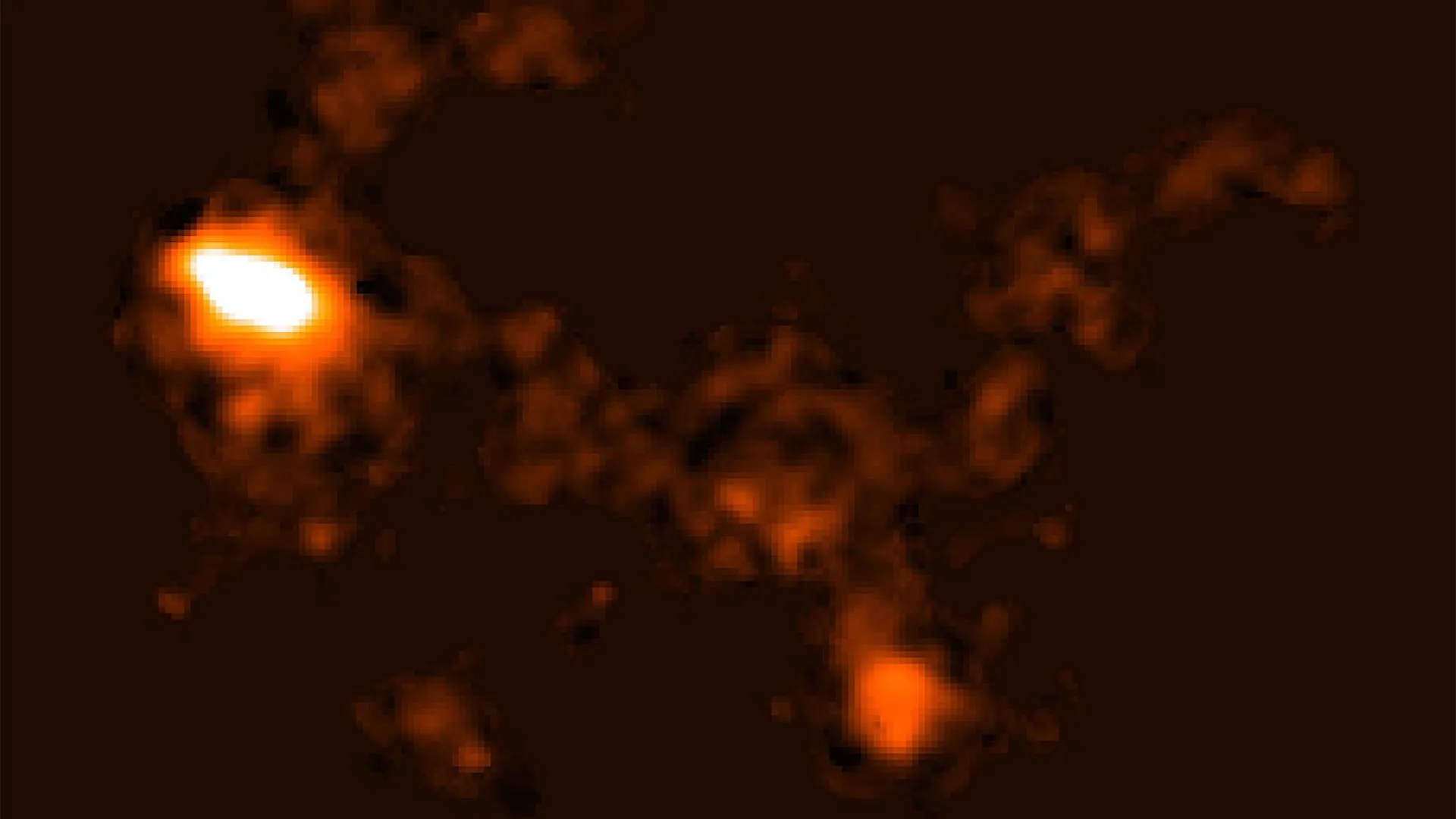
Scientists at The University of Western Australia’s node of the International Centre for Radio Astronomy Research (ICRAR) have made a remarkable discovery: a massive structure stretching about 185,000 light-years between two galaxies, NGC 4532…

Scientists at The University of Western Australia’s node of the International Centre for Radio Astronomy Research (ICRAR) have made a remarkable discovery: a massive structure stretching about 185,000 light-years between two galaxies, NGC 4532…

Scientists have recently captured a rare look at the harsh winter conditions swirling above Mars’ north pole. Inside the planet’s polar vortex, temperatures drop dramatically — much colder than the air outside — and the continuous darkness of…
If you wish you could live in the world of Fallout: New Vegas, then you may need Doc Mitchell to take a look at your brain.
However, if the allure of life on the irradiated New Vegas Strip appeals to you that much, you can get a little taste (this…
Relacorilant plus nab-paclitaxel (Abraxane) produced a progression-free survival (PFS) benefit vs nab-paclitaxel alone in a subgroup of patients with platinum-resistant ovarian cancer (PROC) who had received prior PARP inhibitor treatment, including those who had progressed during PARP inhibitor treatment, according to findings from a pre-planned subgroup analysis of the phase 3 ROSELLA trial (NCT05257408), which were presented at the
In the subgroup of patients who had prior exposure to a PARP inhibitor, relacorilant plus nab-paclitaxel (n = 114) elicited a median blinded independent central review (BICR)–assessed PFS of 7.36 months (95% CI, 5.59-8.18) vs 4.63 months (95% CI, 3.55-5.72) with nab-paclitaxel alone (n = 120; HR, 0.60; 95% CI, 0.42-0.85; nominal P = .0035). The investigator-assessed overall response rates (ORRs) in these respective groups were 39.5% and 30.8%.
Furthermore, in the subgroup of patients who had progressed on a prior PARP inhibitor, the BICR-assessed median PFS with relacorilant plus nab-paclitaxel (n = 86) was 7.36 months (95% CI, 5.39-8.44) vs 3.94 months (95% CI, 3.32-5.72) with nab-paclitaxel alone (n = 97; HR, 0.56; 95% CI, 0.37-0.84; nominal P = .0046). The investigator-assessed ORRs in these respective groups were 34.9% and 26.8%.
“Consistent benefit was reported in this subgroup analysis in PARP [inhibitor]–pretreated patients [with] relacorilant plus nab-paclitaxel,” presenting author Domenica Lorusso, MD, PhD, said.
Lorusso is director of the Gynaecological Oncology Unit at Humanitas Hospital San Pio X, as well as a full professor of obstetrics and gynecology at Humanitas University, Rozzano, in Milan, Italy.
Ovarian cancers harbor glucocorticoid receptor expression, which is a marker of poor prognosis. Relacorilant is a novel, selective glucocorticoid receptor antagonist that restores cancer sensitivity to cytotoxic chemotherapy.
ROSELLA enrolled patients with epithelial ovarian, primary peritoneal, or fallopian tube cancer who had an ECOG performance status of 0 or 1, had progressed less than 6 months after their last dose of platinum therapy, and had received 1 to 3 prior lines of therapy, including prior bevacizumab. Patients were randomly assigned 1:1 to receive nab-paclitaxel at 80 mg/m2 on days 1, 8, 15 of each 28-day cycle, in combination with relacorilant at 150 mg on the day before, the day of, and the day after nab-paclitaxel infusion; or nab-paclitaxel monotherapy at 100 mg/m2 on the same nab-paclitaxel dosing schedule.
PFS by BICR and overall survival (OS) served as the dual primary end points. Secondary end points included investigator-assessed PFS, ORR, duration of response, clinical benefit rate, and safety.
Previously, data presented at the
The addition of relacorilant to nab-paclitaxel also showed a trend toward improved OS among patients who had received a prior PARP inhibitor, although these data were only at 50% maturity at the time of this interim analysis.1 The median OS was 15.61 months (95% CI, 12.02-not reached) with relacorilant plus nab-paclitaxel vs 12.58 months (95% CI, 10.09-15.18) with nab-paclitaxel alone (HR, 0.77; 95% CI, 0.53-1.13; nominal P = .1834).
Lorusso noted that relacorilant plus nab-paclitaxel continued to be well tolerated in the prior PARP inhibitor subgroup. Any treatment-emergent adverse effects (TEAEs) were observed in all patients in this subgroup. Among safety-evaluable patients with prior PARP inhibitor exposure who received relacorilant plus nab-paclitaxel, grade 3 or higher TEAEs were seen in 71.1%, and serious AEs were reported in 31.6%. TEAE-related dose reductions of relacorilant (7.0%), dose reductions of nab-paclitaxel (46.5%), treatment interruptions (72.8%), and treatment discontinuations (8.8%) were also observed.
Among safety-evaluable patients with prior PARP inhibitor exposure who received nab-paclitaxel alone (n = 117), grade 3 or higher TEAEs were seen in 64.1%, and serious AEs were reported in 21.4%. TEAE-related dose reductions of nab-paclitaxel (29.1%), treatment interruptions (58.1%), and treatment discontinuations (6.8%) were also observed.
“The safety profile in the trial subgroup was very similar to that [seen in] the overall population,” Lorusso concluded.
Disclosures: Lorusso reported receiving grants from or having contracts with AstraZeneca, Clovis, Genmab, GSK, Immunogen, Incyte, MSD, Novartis, PharmaMar, Seagen, and Roche; receiving consulting fees from AstraZeneca, Clovis Oncology, Genmab, GSK, Immunogen, MSD, PharmaMar, Seagen, and Novartis; receiving payment or honoraria from AstraZeneca, Clovis, Corcept, Genmab, GSK, Immunogen, MSD, Oncoinvest, PharmaMar, Seagen, and Sutro; receiving support for attending meetings and/or travel from GSK, AstraZeneca, Clovis, and MSD; and participating on Data Safety Monitoring or Advisory Boards for AstraZeneca, Clovis, Corcept, Genmab, GSK, Immunogen, MSD, Oncoinvest, PharmaMar, Seagen, and Sutro.
First Fergana Peace Forum was organized from 15 to 16 October 2025 at Fergana university. More than 300 participants from Central Asia, the CIS, Europe, Asia and the America gathered to discuss under the title “Uniting efforts for peace…