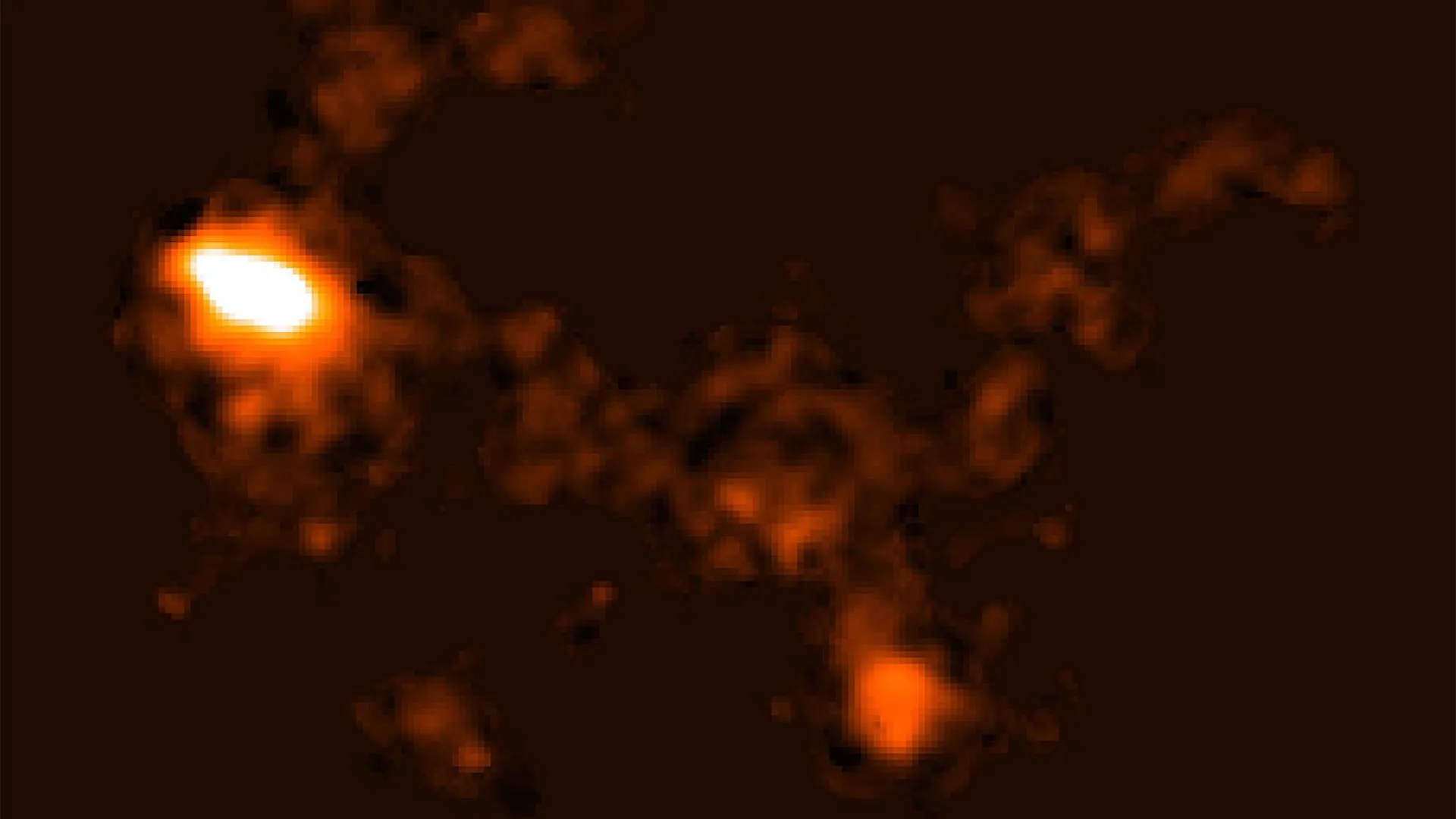
Scientists at The University of Western Australia’s node of the International Centre for Radio Astronomy Research (ICRAR) have made a remarkable discovery: a massive structure stretching about 185,000 light-years between two galaxies, NGC 4532…
Blog
-
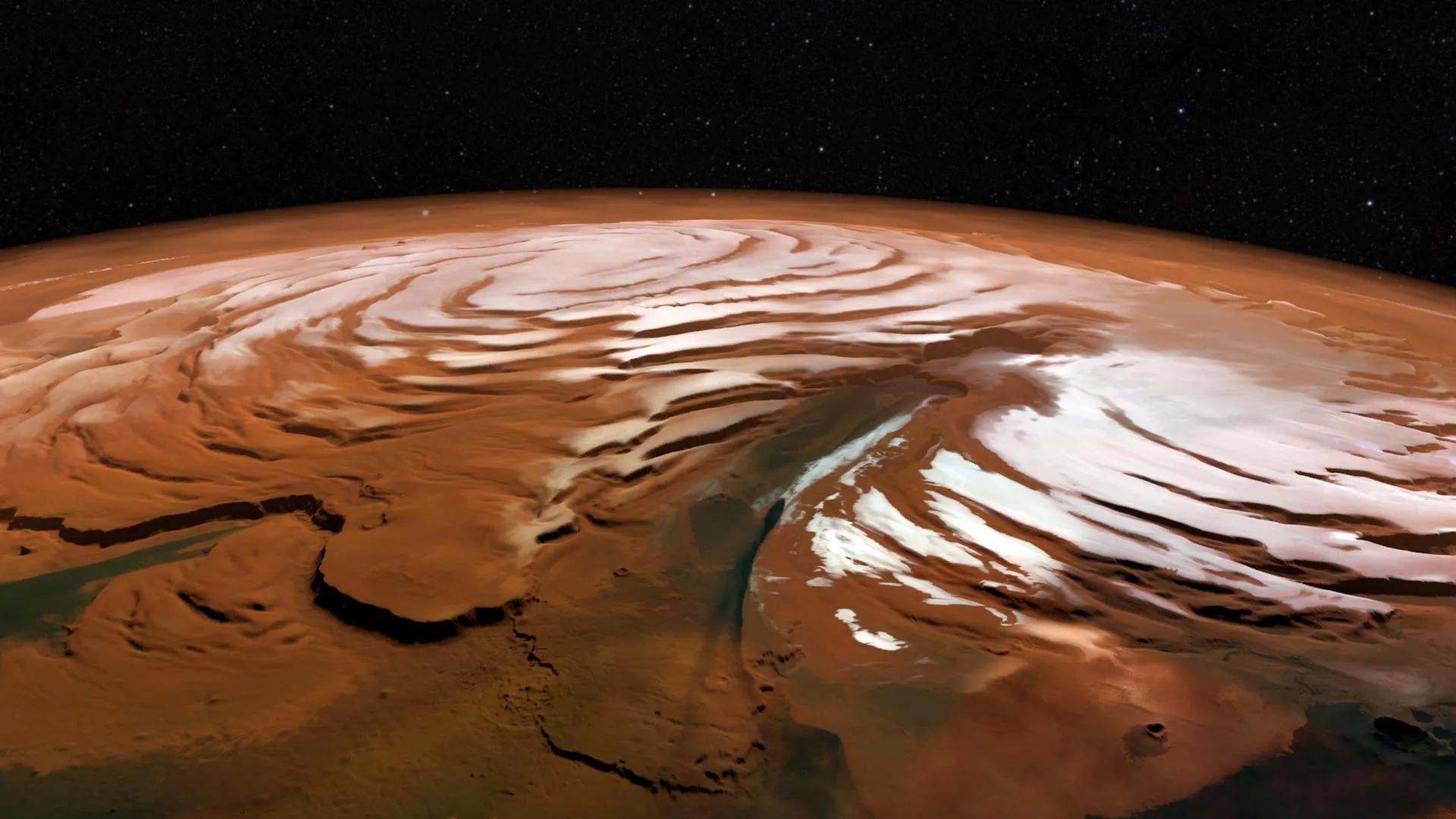
A clue to ancient life? What scientists found inside Mars’ frozen vortex
Scientists have recently captured a rare look at the harsh winter conditions swirling above Mars’ north pole. Inside the planet’s polar vortex, temperatures drop dramatically — much colder than the air outside — and the continuous darkness of…
Continue Reading
-
Fallout New Vegas Surprise Return Stuns Gamers As Sell Out Guaranteed
If you wish you could live in the world of Fallout: New Vegas, then you may need Doc Mitchell to take a look at your brain.
However, if the allure of life on the irradiated New Vegas Strip appeals to you that much, you can get a little taste (this…
Continue Reading
-
Relacorilant Plus Nab-Paclitaxel Yields PFS Advantage in PARP Inhibitor–Exposed PROC
Relacorilant plus nab-paclitaxel (Abraxane) produced a progression-free survival (PFS) benefit vs nab-paclitaxel alone in a subgroup of patients with platinum-resistant ovarian cancer (PROC) who had received prior PARP inhibitor treatment, including those who had progressed during PARP inhibitor treatment, according to findings from a pre-planned subgroup analysis of the phase 3 ROSELLA trial (NCT05257408), which were presented at the
2025 ESMO Congress .1In the subgroup of patients who had prior exposure to a PARP inhibitor, relacorilant plus nab-paclitaxel (n = 114) elicited a median blinded independent central review (BICR)–assessed PFS of 7.36 months (95% CI, 5.59-8.18) vs 4.63 months (95% CI, 3.55-5.72) with nab-paclitaxel alone (n = 120; HR, 0.60; 95% CI, 0.42-0.85; nominal P = .0035). The investigator-assessed overall response rates (ORRs) in these respective groups were 39.5% and 30.8%.
Furthermore, in the subgroup of patients who had progressed on a prior PARP inhibitor, the BICR-assessed median PFS with relacorilant plus nab-paclitaxel (n = 86) was 7.36 months (95% CI, 5.39-8.44) vs 3.94 months (95% CI, 3.32-5.72) with nab-paclitaxel alone (n = 97; HR, 0.56; 95% CI, 0.37-0.84; nominal P = .0046). The investigator-assessed ORRs in these respective groups were 34.9% and 26.8%.
“Consistent benefit was reported in this subgroup analysis in PARP [inhibitor]–pretreated patients [with] relacorilant plus nab-paclitaxel,” presenting author Domenica Lorusso, MD, PhD, said.
Lorusso is director of the Gynaecological Oncology Unit at Humanitas Hospital San Pio X, as well as a full professor of obstetrics and gynecology at Humanitas University, Rozzano, in Milan, Italy.
What Were the Rationale and Design of the ROSELLA Trial?
Ovarian cancers harbor glucocorticoid receptor expression, which is a marker of poor prognosis. Relacorilant is a novel, selective glucocorticoid receptor antagonist that restores cancer sensitivity to cytotoxic chemotherapy.
ROSELLA enrolled patients with epithelial ovarian, primary peritoneal, or fallopian tube cancer who had an ECOG performance status of 0 or 1, had progressed less than 6 months after their last dose of platinum therapy, and had received 1 to 3 prior lines of therapy, including prior bevacizumab. Patients were randomly assigned 1:1 to receive nab-paclitaxel at 80 mg/m2 on days 1, 8, 15 of each 28-day cycle, in combination with relacorilant at 150 mg on the day before, the day of, and the day after nab-paclitaxel infusion; or nab-paclitaxel monotherapy at 100 mg/m2 on the same nab-paclitaxel dosing schedule.
PFS by BICR and overall survival (OS) served as the dual primary end points. Secondary end points included investigator-assessed PFS, ORR, duration of response, clinical benefit rate, and safety.
What Data Have Been Previously Reported From ROSELLA?
Previously, data presented at the
2025 ASCO Annual Meeting showed that the addition of relacorilant to nab-paclitaxelextended median PFS by BICR compared with nab-paclitaxel alone across the entire population of patients with PROC (HR, 0.70; 95% CI, 0.54-0.91; log-rank P = .0076).1,2 Furthermore, data from the interim OS analysis showed that the addition of relacorilant generated a clinically meaningful median OS improvement across the full analysis set, at 16.0 months vs 11.5 months (HR, 0.69; 95% CI, 0.52-0.92; nominal log-rank P = .0121).What Additional Efficacy Data Were Seen in the Analysis of Prior PARP Inhibitor–Exposed Patients in ROSELLA?
The addition of relacorilant to nab-paclitaxel also showed a trend toward improved OS among patients who had received a prior PARP inhibitor, although these data were only at 50% maturity at the time of this interim analysis.1 The median OS was 15.61 months (95% CI, 12.02-not reached) with relacorilant plus nab-paclitaxel vs 12.58 months (95% CI, 10.09-15.18) with nab-paclitaxel alone (HR, 0.77; 95% CI, 0.53-1.13; nominal P = .1834).
What Was the Safety Profile of Relacorilant Plus Nab-Paclitaxel in Patients in ROSELLA Who Had Received Prior PARP Inhibition?
Lorusso noted that relacorilant plus nab-paclitaxel continued to be well tolerated in the prior PARP inhibitor subgroup. Any treatment-emergent adverse effects (TEAEs) were observed in all patients in this subgroup. Among safety-evaluable patients with prior PARP inhibitor exposure who received relacorilant plus nab-paclitaxel, grade 3 or higher TEAEs were seen in 71.1%, and serious AEs were reported in 31.6%. TEAE-related dose reductions of relacorilant (7.0%), dose reductions of nab-paclitaxel (46.5%), treatment interruptions (72.8%), and treatment discontinuations (8.8%) were also observed.
Among safety-evaluable patients with prior PARP inhibitor exposure who received nab-paclitaxel alone (n = 117), grade 3 or higher TEAEs were seen in 64.1%, and serious AEs were reported in 21.4%. TEAE-related dose reductions of nab-paclitaxel (29.1%), treatment interruptions (58.1%), and treatment discontinuations (6.8%) were also observed.
“The safety profile in the trial subgroup was very similar to that [seen in] the overall population,” Lorusso concluded.
Disclosures: Lorusso reported receiving grants from or having contracts with AstraZeneca, Clovis, Genmab, GSK, Immunogen, Incyte, MSD, Novartis, PharmaMar, Seagen, and Roche; receiving consulting fees from AstraZeneca, Clovis Oncology, Genmab, GSK, Immunogen, MSD, PharmaMar, Seagen, and Novartis; receiving payment or honoraria from AstraZeneca, Clovis, Corcept, Genmab, GSK, Immunogen, MSD, Oncoinvest, PharmaMar, Seagen, and Sutro; receiving support for attending meetings and/or travel from GSK, AstraZeneca, Clovis, and MSD; and participating on Data Safety Monitoring or Advisory Boards for AstraZeneca, Clovis, Corcept, Genmab, GSK, Immunogen, MSD, Oncoinvest, PharmaMar, Seagen, and Sutro.
References
- Lorusso D, Quesada S, Chan JK, et al. ROSELLA (GOG3073, ENGOTov72, APGOT-OV10): relacorilant + nab-paclitaxel in the subgroup of patients with platinum-resistant ovarian cancer (PROC) previously exposed to a PARP inhibitor. Presented at: 2025 ESMO Congress; October 17-21, 2025; Berlin, Germany. Abstract LBA45.
- Olawaiye A, Gladieff L, Gilbert L, et al. ROSELLA: a phase 3 study of relacorilant in combination with nab-paclitaxel versus nab-paclitaxel monotherapy in patients with platinum-resistant ovarian cancer (GOG-3073, ENGOT-ov72). J Clin Oncol. 2025;43(suppl 17):LBA5507. doi:10.1200/JCO.2025.43.17_suppl.LBA5507
Continue Reading
-
Sinner, Fritz, Shelton headline Vienna & Basel fields in potentially crucial week in Live Race – ATP Tour
- Sinner, Fritz, Shelton headline Vienna & Basel fields in potentially crucial week in Live Race ATP Tour
- Sinner in line for Bublik rematch in Vienna, Musetti & Tsitsipas in R1 showdown ATP Tour
- Sinner vs. Altmaier Prediction at the Erste Bank Open…
Continue Reading
-
OSCE Supports Dialogue and Unity at the First Fergana Peace Forum
First Fergana Peace Forum was organized from 15 to 16 October 2025 at Fergana university. More than 300 participants from Central Asia, the CIS, Europe, Asia and the America gathered to discuss under the title “Uniting efforts for peace…
Continue Reading
-

KP health department launches probe into Marian Dengue deaths
– Advertisement –
PESHAWAR, Oct 19 (APP):The Khyber Pakhtunkhwa health department has ordered a comprehensive investigation into the alleged dengue-related deaths of two patients at a hospital in Mardan.
The directive, issued by Secretary of…
Continue Reading
-
Final Analysis of COMPASSION-15 Presented at ESMO 2025
HONG KONG, Oct. 19, 2025 /PRNewswire/ — On October 19, 2025, Akeso (9926.HK) announced the final analysis results from the COMPASSION-15/AK104-302 study at the 2025 European Society of Medical Oncology Congress (ESMO 2025) . COMPASSION-15 is a Phase III clinical trial evaluating cadonilimab, Akeso’s first-in-class PD-1/CTLA-4 bispecific antibody, in combination with oxaliplatin and capecitabine as first-line treatment for unresectable, locally advanced, recurrent, or metastatic gastric or gastroesophageal junction (G/GEJ) adenocarcinoma. Professor Shen Lin, principal investigator from Peking University Cancer Hospital, presented the findings in an oral session at ESMO 2025.
In this final analysis presented at ESMO 2025 (median follow-up: 33.9 months), the cadonilimab regimen demonstrated enhanced long-term survival benefits in first-line advanced G/GEJ adenocarcinoma. With the extended follow-up period and more mature data, the cadonilimab treatment regimen showed a further reduction in the risk of death compared to the control group. This consistent benefit was observed across all PD-L1 expression subgroups.
The interim analysis of COMPASSION-15, with a median follow-up of 18.7 months, was previously published in Nature Medicine in January 2025. The data presented at ESMO 2025 were analyzed using the same statistical methodology.
COMPASSION-15 2025 ESMO Data
In the intent-to-treat (ITT) population:
- With long-term follow-up, the cadonilimab regimen demonstrated a significant 39% reduction in the risk of death (OS HR 0.61) versus the control group, showing further improvement over the data from the median 18.7-month follow-up (OS HR 0.66).
- With extended follow-up, in the PD-L1 CPS ≥5 population, the cadonilimab regimen demonstrated a significant 51% reduction in the risk of death (OS HR 0.49; p < 0.001) compared to the control group, showing further improvement over the data from the median 18.7-month follow-up (OS HR 0.58).
- With long-term follow-up, in the PD-L1 CPS <5 population, the cadonilimab regimen showed a significant 24% reduction in the risk of death (OS HR 0.76; 95% CI: 0.59-0.99; p = 0.019) versus the control group, with a strengthening trend of benefit compared to the data from the median 18.7-month follow-up (OS HR 0.75; 95% CI: 0.56-1.00).
- Following extended the follow-up period, the cadonilimab combination regimen maintained a favorable safety profile, with no new safety signals emerging.
In the COMPASSION-15 study, patients with PD-L1 CPS <5 (low expression) and CPS <1 (negative expression) are 49.8% and 23%, respectively, of the ITT population. This represents a higher proportion of PD-L1 low and negative patient population in COMPASSION-15 compared to previous Phase III trials of other immune checkpoint inhibitors used in the treatment of first-line gastric cancer. Previous studies have shown limited responses to PD-1/L1 inhibitors in PD-L1 low-expression or negative patients.
Cadonilimab was approved by the NMPA in September 2024 for the first-line treatment for advanced gastric cancer, offering a new and effective immunotherapy option. Cadonilimab has been included in the 2025 CSCO Gastric Cancer Guidelines as the only Category I recommendation (Level 1A evidence) for first-line immunotherapy, regardless of PD-L1 expression, and is currently widely used in clinical practice.
Forward-Looking Statement of Akeso, Inc.
This announcement by Akeso, Inc. (9926.HK, “Akeso”) contains “forward-looking statements”. These statements reflect the current beliefs and expectations of Akeso’s management and are subject to significant risks and uncertainties. These statements are not intended to form the basis of any investment decision or any decision to purchase securities of Akeso. There can be no assurance that the drug candidate(s) indicated in this announcement or Akeso’s other pipeline candidates will obtain the required regulatory approvals or achieve commercial success. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements.Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of pharmaceutical industry regulation and health care legislation in P.R.China, the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; Akeso’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of the Akeso’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions.
Akeso does not undertake any obligation to publicly revise these forward-looking statements to reflect events or circumstances after the date hereof, except as required by law.
About Akeso
Akeso (HKEX: 9926.HK) is a leading biopharmaceutical company committed to the research, development, manufacturing and commercialization of the world’s first or best-in-class innovative biological medicines. Founded in 2012, the company has created a unique integrated R&D innovation system with the comprehensive end-to-end drug development platform (ACE Platform) and bi-specific antibody drug development technology (Tetrabody) as the core, a GMP-compliant manufacturing system and a commercialization system with an advanced operation mode, and has gradually developed into a globally competitive biopharmaceutical company focused on innovative solutions. With fully integrated multi-functional platform, Akeso is internally working on a robust pipeline of over 50 innovative assets in the fields of cancer, autoimmune disease, inflammation, metabolic disease and other major diseases. Among them, 24 candidates have entered clinical trials (including 15 bispecific/multispecific antibodies and bispecific ADCs. Additionally, 7 new drugs are commercially available. Through efficient and breakthrough R&D innovation, Akeso always integrates superior global resources, develops the first-in-class and best-in-class new drugs, provides affordable therapeutic antibodies for patients worldwide, and continuously creates more commercial and social values to become a global leading biopharmaceutical enterprise.For more information, please visit https://www.akesobio.com/en/about-us/corporate-profile/ and follow us on Linkedin.
SOURCE Akeso, Inc.

Continue Reading
-
Interior minister briefs PM Shehbaz on law and order situation – Dawn
- Interior minister briefs PM Shehbaz on law and order situation Dawn
- TLP leadership to face music: Naqvi The Express Tribune
- No one will be allowed to spread unrest, warn federal ministers The Nation (Pakistan )
- Naqvi meets Owais Noorani Daily…
Continue Reading