A24’s $104 million grossing romance movie, The Materialists, is debuting on HBO Max on Nov. 7 with a linear premiere of Saturday, Nov. 8, 8PM EST.
In the Celine Song directed and written feature, Dakota Johnson plays a young,…

A24’s $104 million grossing romance movie, The Materialists, is debuting on HBO Max on Nov. 7 with a linear premiere of Saturday, Nov. 8, 8PM EST.
In the Celine Song directed and written feature, Dakota Johnson plays a young,…

In 2019, a KFF Health News investigation reported that the Food and Drug Administration had for some 20 years allowed medical device makers to report adverse events — from minor to fatal — on certain products to a database that wasn’t public and that few people even knew existed.
This secret system, known as Alternative Summary Reporting, or ASR, grew to 6 million reports of adverse events alongside continuing operation of the FDA’s public database for such bad news, Manufacturer and User Facility Device Experience, known as MAUDE.
Once KFF Health News revealed its existence, the FDA terminated the secret filing system and made public the entire ASR database, filling in what had been very patchy disclosure of some medical device problems. Filings to ASR had represented about two-thirds of all reported malfunctions and injuries involving approved medical devices between 1997 and 2019.
In a working paper, University of Utah’s Colleen Cunningham and UCLA Anderson’s Jennifer Kao assess how this sudden and massive increase in transparency affected innovation in the medical device industry. Cunningham and Kao study two mechanisms at work as the industry came to grips with actual disclosure:
The transparency altered the quantity and quality of innovation efforts. After the release, the number of applications for new devices fell about 19% in product markets that had at least one incident reported through ASR, Cunningham and Kao report. This occurred across about 100 product categories, including pacemaker electrodes, various catheters, surgical staplers and popular types of breast prostheses.
The quality and safety of new medical devices, meanwhile, got better. Device applications that were submitted in the years following the release typically included stronger safety features and larger technological advancements, the authors show.
The numerical decline is mainly due to manufacturers pivoting away from newly scrutinized product categories, according to Cunningham and Kao’s analysis. The decisions appear driven by the revelations that competitors were having issues with specific products, rather than embarrassment or liability fears surrounding negative exposure around their own devices, the study suggests. With an understanding of more than just their own device problems, firms appeared to reassess potential market risks of the problematic devices, and redeployed research and development funds to other products.
At the same time, product introductions appeared to be different after the data release. New players may have been surprised by all of the adverse events that came to light with the FDA’s disclosure. As incumbents withdrew, these newcomers faced less competition and could use the new data to inform their product development decisions. After some years, these new entrants introduced safer, higher quality devices to fill the void.
The data dump also appeared to affect development of products that were technologically similar to, but not a part of, the disclosure, the study finds. (These products have “predicate relationships,” in FDA terms. They may, for example, use similar components.) Cunningham and Kao found reduced research and development funding in this category after the data release.

Idiopathic MH is distinguished by a full-thickness defect with the…

The ladies of MomTok are back, and they’re just as over each other as they were last season.
Hulu released the Secret Lives of Mormon Wives season three trailer Wednesday morning (below), and the in-fighting and drama of season two is…

Every superhero — or antihero — needs a sidekick. And it turns out that Beetlejuice Betelgeuse does indeed have one! The red supergiant star found in the constellation Orion has captivated stargazers for millennia, and while scientists have…

Ahead of a new era of Formula 1 in 2026, Italian engineer Enrico Cardile moved from Ferrari to Aston Martin. Working alongside Adrian Newey as the team’s Chief Technical Officer, his aim is to turn Aston into winners.
Enrico tells host Beyond…
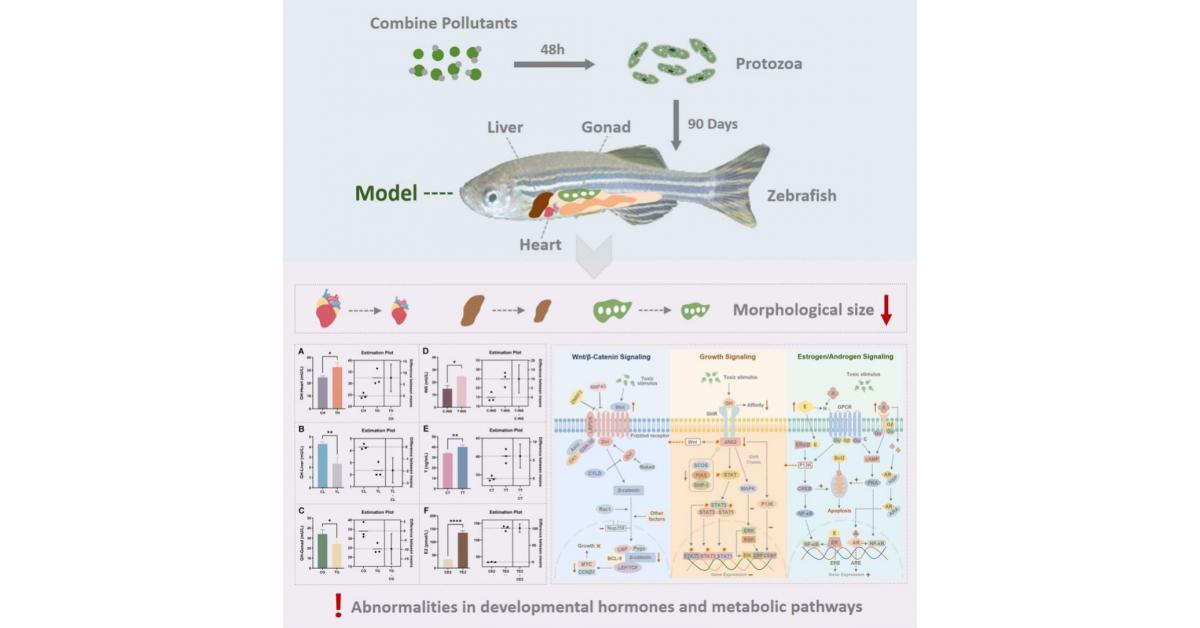
Potential developmental threats of long-term combined pollutant exposure to higher trophic levels. CREDIT: The AUTHORS
GA, UNITED STATES, October 22, 2025 /EINPresswire.com/ — Tiny aquatic organisms can pass microplastics and heavy metals…
Light-to-moderate alcohol consumption is associated with increases in blood pressure (BP) and stopping drinking – even drinking less – may lead to clinically meaningful BP reductions, according to a study published today…

In 2019, the designer Grace Wales Bonner told an interviewer that it was a dream of hers “to work with a brand like Hermès”. Six years later, the 35-year-old Briton was named creative director of menswear for the French luxury company,…