This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Blog
-
Just a moment…
Just a moment… -
Just a moment…
Just a moment… This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Continue Reading
-
Just a moment…
Just a moment… This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Continue Reading
-

TechnipFMC Announces Quarterly Dividend and Additional $2 Billion Share Repurchase Authorization
NEWCASTLE & HOUSTON, October 22, 2025 — TechnipFMC plc (NYSE: FTI) today announced that its Board of Directors has authorized and declared a quarterly cash dividend of $0.05 per share, payable on December 3, 2025 to shareholders of record as of the close of business on the New York Stock Exchange on November 18, 2025, which is also the ex-dividend date.
The Board has also authorized additional share repurchases of up to $2 billion. Together with the remaining balance under the existing program, the Company is now authorized to repurchase shares of up to $2.3 billion, representing nearly 16 percent of the Company’s outstanding shares at the closing price on October 21, 2025.
Doug Pferdehirt, TechnipFMC’s Chair and CEO, stated, “Since the initial share repurchase authorization in July 2022, the Company has returned more than $1.6 billion to shareholders through stock repurchases and dividends. This significant increase to the share authorization exemplifies our confidence in the outlook, as well as our commitment to maximize shareholder value.”
The Company expects to repurchase shares from time to time through open market purchases, privately negotiated transactions, Rule 10b5-1 plans, and any other means in accordance with applicable securities laws. The share repurchase program does not obligate the Company to repurchase shares and may be suspended or discontinued at any time at the Company’s discretion.
Important Information for Investors and Securityholders
Forward-Looking Statement
This release contains “forward-looking statements” as defined in Section 27A of the United States Securities Act of 1933, as amended, and Section 21E of the United States Securities Exchange Act of 1934, as amended. The words “expect,” “believe,” “estimated,” and other similar expressions are intended to identify forward-looking statements, which are generally not historical in nature. Such forward-looking statements involve significant risks, uncertainties and assumptions that could cause actual results to differ materially from our historical experience and our present expectations or projections. For information regarding known material factors that could cause actual results to differ from projected results, including our assumptions and projections regarding the announced share repurchase program, please see our risk factors set forth in our filings with the United States Securities and Exchange Commission, which include our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K. We caution you not to place undue reliance on any forward-looking statements, which speak only as of the date hereof. We undertake no obligation to publicly update or revise any of our forward-looking statements after the date they are made, whether as a result of new information, future events or otherwise, except to the extent required by law.
###
About TechnipFMC
TechnipFMC is a leading technology provider to the traditional and new energy industries, delivering fully integrated projects, products, and services.
With our proprietary technologies and comprehensive solutions, we are transforming our clients’ project economics, helping them unlock new possibilities to develop energy resources while reducing carbon intensity and supporting their energy transition ambitions.
Organized in two business segments — Subsea and Surface Technologies — we will continue to advance the industry with our pioneering integrated ecosystems (such as iEPCI™, iFEED™ and iComplete™), technology leadership and digital innovation.
Each of our approximately 21,000 employees is driven by a commitment to our clients’ success, and a culture of strong execution, purposeful innovation, and challenging industry conventions.
TechnipFMC uses its website as a channel of distribution of material company information. To learn more about how we are driving change in the industry, go to www.TechnipFMC.com and follow us on X @TechnipFMC.
Contacts
Investor relations
Matt Seinsheimer
Senior Vice President, Investor Relations and Corporate Development
Tel: +1 281 260 3665
Email: Matt SeinsheimerJames Davis
Director, Investor Relations
Tel: +1 281 260 3665
Email: James DavisMedia relations
Mendi Head
Public Relations and Media Relations
Tel: +1 346 297 8392
Email: Mendi HeadContinue Reading
-

PacBio to Report Third Quarter 2025 Financial Results on November 5, 2025
MENLO PARK, Calif., Oct. 22, 2025 (GLOBE NEWSWIRE) — PacBio (NASDAQ: PACB) announced today that it will hold its quarterly conference call to discuss its third quarter 2025 financial results on Wednesday, November 5, 2025, at 4:30 pm Eastern Time.
The call will be webcast and may be accessed at PacBio’s website at https://investor.pacificbiosciences.com/.
Date: Wednesday, November 5, 2025, at 4:30 pm ET (1:30 pm PT)
Listen live via internet or replay: https://investor.pacificbiosciences.com/
Toll-free: 1-888-349-0136
International: 1-412-317-0459If using the dial-in option, please join the call ten minutes before the start time using the appropriate number above and ask to join the “PacBio Q3 Earnings Call.”
About PacBio
PacBio (NASDAQ: PACB) is a premier life science technology company that designs, develops, and manufactures advanced sequencing solutions to help scientists and clinical researchers resolve genetically complex problems. Our products and technologies, which include our HiFi long-read sequencing, address solutions across a broad set of research applications including human germline sequencing, plant and animal sciences, infectious disease and microbiology, oncology, and other emerging applications. For more information, please visit www.pacb.com and follow @PacBio.
PacBio products are provided for Research Use Only. Not for use in diagnostic procedures.
Contacts
Investors:
Jim Gibson
ir@pacb.comMedia:
pr@pacb.comContinue Reading
-

The Story Behind Ireland’s First Win Over the All Blacks » allblacks.com
Playing the All Blacks in Chicago again on Saturday week has fond memories for Irish rugby fans, as their first game resulted in Ireland’s first win over New Zealand.
Donnacha Ryan played in the 40-29 win and told The Irish…
Continue Reading
-

Real-World Studies Reinforce Axi-Cel and Liso-Cel as Effective LBCL Treatments
How have the FDA approvals of axi-cel and liso-cel affected the LBCL treatment paradigm?
Five years ago, irrespective of relapse status or primary refractoriness, the standard of care would have been salvage platinum-containing chemotherapy…
Continue Reading
-

$45 M. Basquiat, $15 M. Kerry James Marshall Consigned by Top Collectors
With the London sales all wrapped and Art Basel Paris kicking off earlier this week, there’s only one major art world event left on the calendar between now and Thanksgiving—and it’s a big one: the November marquee auctions.
The…
Continue Reading
-
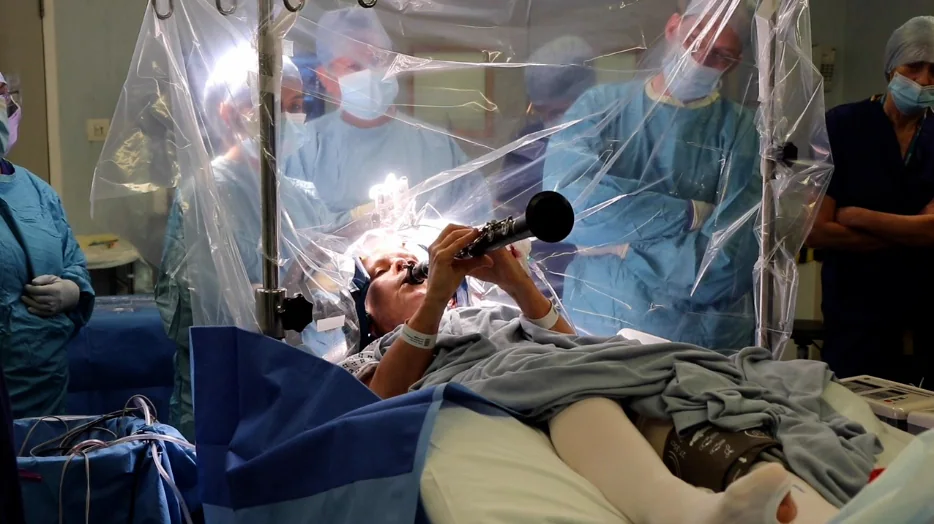
A Parkinson’s patient plays clarinet during Deep Brain Stimulation surgery – The Washington Post
- A Parkinson’s patient plays clarinet during Deep Brain Stimulation surgery The Washington Post
- ‘Delighted’ woman played clarinet in brain surgery BBC
- Denise Bacon underwent a four-hour procedure to improve her Parkinson’s symptoms. The semi…
Continue Reading
-
Drugs found to reduce advanced prostate cancer death rate
A powerful new drug combo has yielded a major breakthrough for men battling an aggressive form of prostate cancer.
Adding the drug enzalutamide to standard hormone therapy reduced the risk of premature death by more than 40% in patients whose…
Continue Reading