Busts by Rodin and works by Kandinksy and Matisse are among some 400 items belonging to the late actor going up for auction next month.
Three Golden Globe statues, a variety of film memorabilia and annotated scripts, posters and an easel are also…

Busts by Rodin and works by Kandinksy and Matisse are among some 400 items belonging to the late actor going up for auction next month.
Three Golden Globe statues, a variety of film memorabilia and annotated scripts, posters and an easel are also…

We’re likely only a few months away from the next Samsung Unpacked event and rumors are swirling about the Galaxy S26 line of phones. And boy, have they been all over the place. A few months ago, there was talk that Samsung would be replacing its…

Squire Patton Boggs has advised GBG, a global identity technology business, on the acquisition of DataTools Pty Limited, a leading provider of address validation and data quality solutions in Australia and New Zealand.
The Squire Patton Boggs team was led by Corporate partners Louisa Hine (Sydney) and Giles Chesher (Manchester).
DataTools is one of the largest providers of address validation and data quality solutions in Australia and New Zealand (ANZ). The acquisition adds scale and deepens GBG’s existing address verification presence in ANZ and is complementary to GBG’s identity verification platform, enhancing its broader proposition in the region.

Recruitment of patients into clinical research studies is a persistent and well-documented challenge []. The number of clinical trials open for patient enrollment has increased dramatically over time, growing from 4000 protocols in 2000 to more than 134,000 in 2023, which has intensified the challenge of patient recruitment []. Approximately 80% of clinical trials are delayed or terminated early because of recruitment problems []. Poor patient recruitment is the top cause of clinical trial delays []. Failure to meet recruitment goals delays treatment advances, threatens internal validity, and raises concerns about the generalizability of results []. There is a clear need to improve recruitment practices.
The integration of electronically collected patient-entered data within clinical practices offers innovative approaches to aid research recruitment [,]. While patient-entered data can help identify potential study participants through eligibility screening and interest assessment, these methods present distinct challenges. Using electronic patient questionnaires to screen for study eligibility, while seemingly straightforward, increases the burden of patient response and can create frustration among patients uninterested in research participation []. This approach also requires ongoing technical resources to maintain and update screening criteria as studies evolve. More problematically, additional recruitment outreach to patients who have declined participation via questionnaire violates patient autonomy—a cornerstone of ethical research []. This practice risks eroding trust in the health care system.
A more nuanced approach involves identifying a patient attribute associated with a higher propensity for research participation. This strategy enables research teams to prioritize outreach efforts efficiently to those with a higher likelihood of participating while avoiding prescribed eligibility criteria or direct elicitation of their interest in research. This method reduces the risk of alienating patients and reduces patient burden of questionnaire completion.
The objectives of this study were to (1) identify patient-reported questions that reflect perceptions about participating in clinical research studies and (2) determine whether patient responses to these questions are predictive of interest in participating in a precision medicine research registry.
This was a mixed methods study that used an exploratory sequential design. It was conducted in 2 phases. Phase 1 was a qualitative analysis that informed the phase 2 quantitative study. In phase 1, cognitive interviews were conducted to identify attribute questions that would reflect the likelihood that patients would participate in a clinical research study. In phase 2, the relationship between patients’ responses to the questions, identified in phase 1, and their interest in learning more about an ongoing research study was assessed in patients seen in primary care clinics. The identified “research perception” questions were added to an electronic questionnaire set that patients routinely complete before primary care office visits. The set also included a question about interest in being contacted about an ongoing precision medicine registry ().
Phase 1 of this study was reviewed by the Cleveland Clinic Institutional Review Board and considered preparatory for research. Participants in phase 1 received an information sheet inviting them to participate in the cognitive interviews, which outlined the study’s purpose, participation requirements, and voluntary nature of their involvement. Each participant received a US $25 Amazon gift card as compensation. Phase 2 received exemption from the Cleveland Clinic Institutional Review Board (IRB 19-465), which determined that informed consent was not required for either phase of the study. Project data were securely stored and analyzed on Cleveland Clinic servers, with access restricted to authorized study personnel.
Patients were recruited through the Cleveland Clinic Healthcare Partners program, which is comprised of patients who volunteer to provide their perspective about the design and delivery of care. Of the 4590 Healthcare Partners members, 1000 (21.79%) were randomly selected and sent an online invitation to participate in a cognitive interview. Of these 1000 patient panel members, 200 (20%) indicated interest in participating. Of these 200 potential participants, 32 (16%) were selected using purposive sampling to obtain a representation of patients across levels of patient-reported health status, sex, age, and race.
After informed consent, 30-minute patient interviews were conducted via videoconference calls between October 31, 2017, and November 14, 2017, to develop self-reported questions that would reflect patients’ likelihood of participating in a clinical research study. All sessions were conducted by an experienced qualitative researcher (RF) and audio recorded and transcribed.
The specific aims of the interviews were to (1) explore the factors that impact the decision to participate in clinical research; (2) evaluate the clarity of, and participant responses to, 9 potential questions; and (3) identify alternative questions that may reflect patients’ likelihood of participating in a research study that were not previously considered. The 9 potential questions were selected based on a literature review of factors considered important in patients’ decisions to participate in clinical research [-] as well as the feasibility of assessment through patient questionnaires. An interview guide was developed () that contained candidate “research perception” questions. Some of the candidate “research perception” questions focused on participants’ overall impression of research, while 2 questions asked about the relevance of potential benefit to the patient versus benefit to others. Several questions asked about different factors that may affect their decision to participate, such as risk, location, and time commitment. Finally, there was a question about the importance of being informed about ongoing research trials for which they would be eligible. Participants were asked to respond to each of the candidate “research perception” questions on a 4-point Likert scale. They were then asked to explain the reasons for their responses.
The study used a deductive and explanatory approach to qualitative analysis. This approach was chosen because it provided a more structured investigation, allowing for a comprehensive understanding of patients’ perspectives on the best question to assess their interest in participating in potential research projects []. Field notes were recorded for each interview and were supplemented by transcripts for verbatim quotes and details. Two qualitative analysts then coded the data for themes and subthemes under the supervision of the senior qualitative researcher (RF). The coding tree was organized into 4 overarching themes that were identified before the start of interviews as part of the research objectives: level of interest to participate and past participation in clinical research at Cleveland Clinic, general perceptions of the importance of clinical research, drivers to participate in clinical research, and areas for improvement to recruit appropriate patients into clinical research. These themes reflect attitudinal motivations that drive patients to participate in clinical research. Subthemes were developed to understand the more nuanced aspects of the data, including the varying drivers for participating in clinical research and recommendations to improve the patient recruitment process. The hierarchical structure of the content analysis was organized from general categories to specific topics. A summary report was developed that included findings with recommendations.
Phase 2 was a cross-sectional cohort study to evaluate whether the “research perception” questions identified in phase 1 could predict patient interest in an ongoing precision medicine research registry. From November 13, 2018, to April 19, 2019, the “research perception” questions from phase 1 were added to the standard patient questionnaires that patients routinely completed through the electronic health record (EHR) patient portal (MyChart [Epic Systems Corporation]) before their primary care visits. As part of the standard of care, patients complete these electronic questionnaires either through the patient portal before arrival or by using tablets and computer workstations after arrival. Patients may decline to complete questionnaires or skip individual questions, and responses are immediately available to health care providers within the EHR.
From May 9, 2017, to April 19, 2019, all patients at select primary care locations also answered a “research recruitment” question asking whether they were interested in being contacted to learn more about a precision medicine registry (). They were asked this question only once. Those who expressed interest selected their preferred contact method (telephone or email) and were subsequently contacted by a research coordinator through a separate process unrelated to this study.
The phase 2 patient population included primary care patients who completed both the “research perception” and “research recruitment” questions during primary care visits between November 13, 2018, and April 19, 2019.
In addition to responding to the “research perception” and “research recruitment” questions, patients completed the Patient-Reported Outcomes Measurement Information System Global Health (PROMIS GH) scale, the Patient Health Questionnaire-2 (PHQ-2) depression screen, and internally developed social needs questions. The PROMIS GH is a widely used, generic health measure comprising 10 items that generate mental and physical health summary scores, with higher scores indicating better health status []. The PHQ-2 includes 2 items, with higher scores reflecting more severe depressive symptoms []. Scores above 3 are indicative of at least moderate depressive symptoms. The social needs questions had binary (yes or no) responses and asked the following: (1) “In the last 12 months, has it been hard for you to pay any of these bills?” (2) “In the last 12 months, have you had to forgo healthcare because you didn’t have a way to get there?” (3) “In the last 3 months, were you ever worried your food would run out before you could buy more?” (4) “In the last 12 months, did you ever sleep in a shelter or not have a steady place to live?”
Additional demographic variables extracted from the EHR included age, sex, race, marital status, and median household income estimated from the 2010 census using zip codes. The authors had full access to the data from the study population.
Descriptive statistics were used to evaluate responses to the “research perception” questions and to compare the characteristics of patients according to their responses to the precision medicine “research recruitment” question. Characteristics were compared by response to the precision medicine “research recruitment” question using the 2-tailed t test for continuous variables and the chi-square test for categorical variables.
Multivariable logistic regression models were constructed to predict response to the “research recruitment” question. Each of the 3 “research perception” questions were included in separate models to evaluate the ability of these questions to independently predict patients’ interest in being contacted to learn more about the ongoing research study if combined with other clinical variables available in the EHR. The independent variable in these models was one of the “research perception” questions. The dependent variable was patient response to the “research recruitment” question. Covariates included age, sex, race, marital status, median household income, PROMIS GH physical and mental health summary scores, depressive symptoms based on the PHQ-2, and a positive response to any of the social needs questions. Interaction effects were also explored between the independent variables and age, sex, and race in separate models. Any significant interaction effects at P<.05 were included in the final models.
The diagnostic accuracy of the 3 “research perception” questions to predict positive response on the “research recruitment” question was assessed using receiver operating characteristic curve analysis. Area under the curve, the Youden index, sensitivity, specificity, negative predictive value, and positive predictive value were calculated for responses of “strongly agree” or “agree” as well as “very important” or “important” on the “research perception” questions. Statistical significance was established throughout at P<.05. All phase 2 analyses were conducted using SAS 9.4 (SAS Institute Inc).
The 32 participants who completed the cognitive interviews had a range of self-reported health conditions. Of the 32 participants, 13 (41%) were female, 13 (41%) were aged 65 years or older, and 11 (34%) had previously participated in a clinical research study ().
| Characteristics | Participants, n (%) | ||
| Age group (y) | |||
| 21-54 | 7 (22) | ||
| 55-64 | 12 (37) | ||
| ≥65 | 13 (41) | ||
| Sex: female | 13 (41) | ||
| Participated in clinical research study at Cleveland Clinic | 11 (34) | ||
| Educational level | |||
| High school graduate | 3 (9) | ||
| Some college or graduated 2-year college | 9 (28) | ||
| Graduated 4-year college | 8 (25) | ||
| Postgraduate degree | 12 (37) | ||
| Race | |||
| Asian | 2 (6) | ||
| Black | 6 (19) | ||
| White | 22 (69) | ||
| Other | 2 (6) | ||
| Marital status | |||
| Married or partnered | 22 (69) | ||
| Single | 4 (12) | ||
| Divorced or widowed | 6 (19) | ||
| Self-reported health condition | |||
| Excellent | 8 (25) | ||
| Good | 8 (25) | ||
| Fair | 11 (34) | ||
| Poor | 5 (16) | ||
aCognitive interviews were performed between October 31, 2017 and November 14, 2017 (for details, refer to the Cognitive Interviews subsection under Phase 1: Qualitative Study to Identify Optimal Question on Perception of Research) to identify a question that would best capture patients’ interest in participating in clinical research.
Data saturation was reached, with participants in the cognitive interviews voicing strong support for clinical research, recognizing its important role in advancing patient care advancement, and agreeing that it should be part of Cleveland Clinic’s mission (). Participants indicated that they preferred receiving information about research opportunities in person directly from their physicians, citing the trust-based relationship with their health care providers and their health care providers’ unique understanding of their medical needs. While participants also viewed emails, MyChart messages, and telephone calls as effective ways to learn about research eligibility, they wanted these to serve as secondary forms of communication after initial discussion with their physicians.
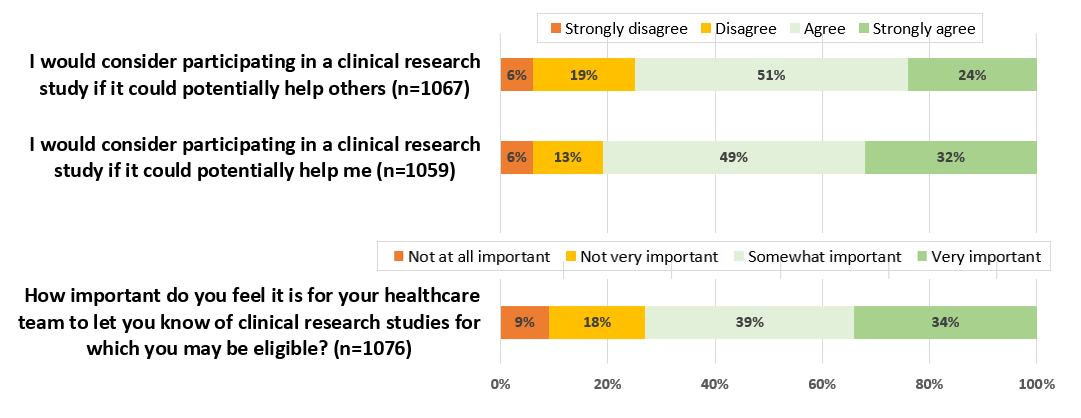
Participants held highly favorable views toward research, with 97% (31/32) agreeing or strongly agreeing that it should be part of the institution’s mission and 100% (32/32) affirming that research enhances patient care. Likewise, nearly all respondents indicated that they would consider participating in research if it benefited them (32/32, 100%) or others (31/32, 97%). Participants indicated that they feel it is important to help others, and many indicated feeling a sense of responsibility to give back; however, they would not participate in a clinical study to help others if they were physically or mentally harmed in the process. Of the 32 participants, 31 (97%) also expressed that it was important or very important to be informed about clinical research opportunities for which they were eligible. Furthermore, a few (2/32, 6%) said that they would be disappointed if their physician did not inform them about clinical studies for which they may be eligible candidates.
There was slightly less consensus on the significance of practical factors such as location, time commitment, and compensation ().
Participants found all questions to be clear and easy to understand, with no suggestions for additional questions. As the most effective question for gauging patients’ interest in research participation, they identified the following: “I would consider participating in a clinical research study if it could potentially help me.” This question, along with 2 other candidates, was chosen for piloting in clinical practice. The selection of these questions was based on participant feedback, the distribution of participant responses, and their perceived relevance. The additional 2 questions were the following: “I would consider participating in a clinical research study if it could potentially help others” and “How important do you feel it is for your healthcare provider to let you know of research trials for which you may be eligible?”
| Questions | Strongly agree, n (%) | Agree, n (%) | Disagree, n (%) | Strongly disagree, n (%) |
| I feel that research should be part of Cleveland Clinic’s mission | 24 (75) | 7 (22) | 1 (3) | 0 (0) |
| I feel that research is important to be able to improve patient care | 28 (88) | 4 (12) | 0 (0) | 0 (0) |
| I would consider participating in a clinical research study if it could potentially help meb | 23 (72) | 9 (28) | 0 (0) | 0 (0) |
| I would consider participating in a clinical research study if it could potentially help othersb | 21 (66) | 10 (31) | 0 (0) | 1 (3) |
aCognitive interviews were conducted between October 31, 2017 and November 14, 2017 (for details, refer to the Cognitive Interviews subsection under Phase 1: Qualitative Study to Identify Optimal Question on Perception of Research).
bThese questions were selected for pilot implementation in electronic questionnaires.
| Questions | Very important, n (%) | Somewhat important, n (%) | Not very important, n (%) | Not at all important, n (%) |
| Level of personal risk for adverse health outcomes | 20 (63) | 9 (28) | 2 (6) | 1 (3) |
| The location of the clinical research study is easily accessible | 13 (50) | 7 (22) | 2 (6) | 7 (22) |
| Whether you would be paid to participate | 1 (3) | 10 (31) | 8 (25) | 13 (41) |
| The amount of time commitment to participate | 12 (41) | 8 (28) | 3 (10) | 6 (21) |
| How important do you feel it is for your healthcare provider to let you know of research trials for which you may be eligible?b | 22 (69) | 9 (28) | 0 (0) | 1 (3) |
aCognitive interviews were performed between October 31, 2017 and November 14, 2017 (for details, refer to the Cognitive Interviews subsection under Phase 1: Qualitative Study to Identify Optimal Question on Perception of Research).
bThis question was selected for pilot implementation in electronic questionnaires.
A total of 1077 patients completed the “research recruitment” question and at least 1 of the 3 “research perception” questions and were included in the study cohort (mean age 48.3, SD 16.3 years; 625/1065 female, 58.68%; 661/1005 White, 65.77%; ). Mean PROMIS GH physical and mental health summary scores were 49.4, SD 8.8 and 49.6, SD 9.6, respectively, similar to the mean of the US general population. At least moderate depressive symptoms were indicated by 11% (106/936) of the patients. Moreover, 14.1% (148/1050) indicated that they had concerns about paying their bills, and 2.57% (27/1051) indicated that they had difficulty with transportation to their medical appointments.
| Characteristics | All patients completing questions | Patients who did not wish to be contacted about the precision medicine registry | Patients who wished to be contacted about the precision medicine registry | P value | |||||||
| Age (y), mean (SD) | 48.3 (16.3) | 47.2 (16.4) | 51.4 (15.6) | <.001 | |||||||
| Female, n (%) | 625/1065 (58.7) | 457/789 (57.9) | 168/276 (60.9) | .35 | |||||||
| Race, n (%) | .96 | ||||||||||
| Black | 254/1005 (25.3) | 191/750 (25.5) | 63/255 (24.7) | ||||||||
| White | 661/1005 (65.8) | 491/750 (65.5) | 170/255 (66.7) | ||||||||
| Other | 90/1005 (9) | 67/750 (8.9) | 23/255 (9) | ||||||||
| Married, n (%) | 530/1047 (50.6) | 394/780 (50.5) | 136/267 (50.9) | .90 | |||||||
| Household income (in units of US $10,000), mean (SD) | 4.80 (2.04) | 4.81 (2.02) | 4.80 (21.1) | .94 | |||||||
| Patient-reported outcomes, mean (SD) | |||||||||||
| PROMIS GHb | |||||||||||
| Physical health | 49.4 (8.8) | 50.4 (8.3) | 46.7 (9.4) | <.001 | |||||||
| Mental health | 49.6 (9.6) | 50.4 (9.4) | 47.3 (9.9) | <.001 | |||||||
| PHQ-2c depression screen | 0.78 (1.34) | 0.68 (1.27) | 1.05 (1.51) | <.001 | |||||||
| Depressed moodd, n (%) | 106/963 (11) | 68/711 (9.6) | 38/252 (15.1) | .02 | |||||||
| Social needs questions, n (%) | |||||||||||
| Payment trouble | 148/1050 (14.1) | 84/777 (10.8) | 64/273 (23.4) | <.001 | |||||||
| Transportation trouble | 27/1051 (2.6) | 11/778 (1.4) | 16/273 (5.9) | <.001 | |||||||
| Food trouble | 431050 (4.1) | 19/777 (2.4) | 24/273 (8.8) | <.001 | |||||||
| Housing trouble | 28/1050 (2.7) | 14/777 (1.8) | 14/273 (5.1) | .003 | |||||||
| Any social needs | 166/1050 (15.8) | 94/777 (12.1) | 72/273 (26.4) | <.001 | |||||||
aPatients seen in primary care clinics completed these electronic patient-reported outcomes as part of the standard of care.
bPROMIS GH: Patient-Reported Outcomes Measurement Information System Global Health.
cPHQ-2: Patient Health Questionnaire-2.
dDepressed mood based on PHQ-2 score of ≥3.
Of the 1077 patients, 278 (25.8%) responded affirmatively to the “research recruitment” question, indicating that they were interested in being contacted about the precision medicine registry. Patients who responded affirmatively were older than those who responded no (mean 51.4, SD 15.6 years vs mean 47.2, SD 16.4 years), had worse self-reported physical and mental health conditions, were more likely to report depressive symptoms (38/252, 15.1% vs 68/708, 9.6%), and indicated more social needs (72/273, 26.4% vs 94/777, 12.1%).
The majority of the patients responded positively to the “research perception” questions: 75% (800/1067) agreed or strongly agreed that they would consider participating in a clinical research study if it could potentially help others, while 81.02% (858/1059) indicated that they would consider participating if it would help them (). In addition, 72.96% (785/1076) of the respondents felt that it was important for their health care team to let them know of clinical research studies for which they may be eligible.
More positive responses to all 3 “research perception” questions were significantly associated with interest in the precision medicine registry. A dose-response relationship was demonstrated for the responses to each question (). For the question “I would consider participating in a clinical research study if it could potentially help others,” responses of “agree” and “strongly agree” were significantly associated with interest in the precision medicine registry (adjusted odds ratio 8.40, 95% CI 2.48-28.5 and adjusted odds ratio 17.6, 95% CI 5.08-61.1, respectively). The multivariable models incorporating demographic and health factors alongside “research perception” questions demonstrated superior discriminative ability compared to models using “research perception” questions alone, with C-statistic values ranging from 0.716 to 0.752 in adjusted models compared to values ranging from 0.628 to 0.650 in unadjusted models (). The highest discrimination was achieved by the model that included the “research perception” question “I would consider participating in a clinical research study if it could potentially help me” (C-statistic=0.752).
| Covariates and response options | Adjusted odds ratio (95% CI) | P value | C-statistic | |||||
| “I would consider participatingin a clinical research study if it could potentially help others”(reference: “strongly disagree”) | 0.746 | |||||||
| Disagree | 2.39 (0.66-8.71) | .19 | ||||||
| Agree | 8.40 (2.48-28.5) | <.001 | ||||||
| Strongly agree | 17.6 (5.08-61.1) | <.001 | ||||||
| “I would consider participating in a clinical research study if it could potentially help me”b(reference: “strongly disagree”) | 0.752 | |||||||
| Disagree | 1.14 (0.29-4.55) | .85 | ||||||
| Agree | 5.50 (1.63-18.5) | .006 | ||||||
| Strongly agree | 11.1 (3.28-37.8) | <.001 | ||||||
| “How important do you feel it is for your healthcare provider to let you know of research trials for which you may be eligible?” (reference: “not at all important”) | 0.716 | |||||||
| Not very important | 2.23 (0.91-5.48) | .28 | ||||||
| Somewhat important | 2.91 (1.26-6.72) | .01 | ||||||
| Very important | 6.36 (2.77-14.6) | <.001 | ||||||
aThe independent variables in these models were the “reception perception” questions, and the dependent variable was interest in the precision medicine research registry. The models adjusted for age, sex, race, marital status, median household income, depressive symptoms, Patient-Reported Outcomes Measurement Information System Global Health, any social needs, and significant interactions.
bSignificant interaction with current age; estimates presented for the model without the interaction effect.
The “research perception” questions demonstrated high sensitivity but limited specificity (). For responses of “agree” or “strongly agree” or “important” or “very important,” sensitivity exceeded 85% across all questions. Similarly high negative predictive values (>85%) indicated that patients who responded negatively to these questions were unlikely to express interest in the precision medicine registry. However, the questions demonstrated low specificity (24%-31%) and positive predictive values (30%), indicating that positive responses were not strong predictors of actual interest in the precision study.
| Variables | “Research perception” questions | ||
| “I would consider participating in a clinical research study if it could potentially help others”: “strongly agree” or “agree” | “I would consider participating in a clinical research study if it could potentially help me”: “strongly agree” or “agree” | “How important do you feel it is for your healthcare provider to let you know of research trials for which you may be eligible?”: “very important” or “important” | |
| AUCa | 0.628 | 0.650 | 0.636 |
| Youden index | 0.183 | 0.186 | 0.158 |
| Sensitivity (%) | 88.4 | 94.6 | 84.9 |
| Specificity (%) | 30 | 24 | 31 |
| PPVb (%) | 30.7 | 30.3 | 30 |
| NPVc (%) | 88 | 92.7 | 85.5 |
aAUC: area under the curve.
bPPV: positive predictive value.
cNPV: negative predictive value.
This study demonstrated that the response to 1 question added to electronic patient questionnaire sets about patient perceptions regarding research may help prioritize recruitment efforts to patients who are more likely to participate in clinical research studies.
There are several potential approaches to using patient-entered data to aid in research recruitment (), with each having distinct advantages and limitations. While methods that directly assess patient eligibility and interest by using screening questions can reduce the research team’s workload, practical constraints within clinical care settings often limit their feasibility and may compromise respect for patient preferences.
| Options | Strengths | Limitations |
| 1. Ask specific eligibility questions for each clinical trial |
|
|
| 2. Ask common eligibility questions that can be used across the clinical trials for each area |
|
|
| 3. Ask about general interest in participating in, or being contacted about, research |
|
|
| 4. Ask general question about perception of research (treat as “patient attribute”) |
|
|
Capturing patients’ attitudes toward research offers a balanced solution that preserves autonomy and prevents the frustration that may occur when those who have explicitly declined contact about research are nonetheless approached. In addition, this approach is concise, straightforward to implement, and requires minimal ongoing maintenance.
The question “I would consider participating in a clinical research study if it could potentially help me” demonstrated superior performance metrics, with both a higher area under the curve and greater sensitivity compared to the alternative questions. However, its direct personal nature may make it less suitable for integration into general clinical questionnaires. Conversely, the question “How important do you feel it is for your healthcare provider to let you know of research trials for which you may be eligible” had the lowest C-statistic value and sensitivity but may have a better contextual fit within standard previsit questionnaires, potentially making it more practical for routine clinical implementation. Specificity was relatively low for all questions; in this context, optimizing sensitivity over specificity can be considered advantageous, as it minimizes the risk of missing potentially interested participants while accepting a higher rate of false positives.
The cognitive interviews revealed that patients prefer to hear about research opportunities directly from their physician. A preference for health care provider to patient discussion about research has been noted by others [-]. Incorporating “research perception” questions within the clinical workflow supports this preference by enabling physicians to prioritize recruitment efforts during their office visit based on patient responses indicating an interest in research. This approach is used by the Movement Disorder Section of the Neurological Institute at our health system, where patients answer the “research perception” question “I think it’s important to be told of research trials for which I may be eligible” alongside other clinical questions before their visit. In the experience of the authors, this screening method has proven effective in prompting clinicians to spend additional time discussing relevant studies with patients who express interest.
Our adjusted models, which contained a “research perception” question and additional variables, had improved discriminative capacity. If electronically implemented, they could allow a more precise estimation of patients’ interest in research. However, targeting participants based on demographic and health status characteristics could introduce greater patient selection bias, reducing a study’s generalizability. Adjusted models would also be more resource intensive to implement.
Recent advances in EHR systems, apart from patient-entered data functionality, have also streamlined the patient recruitment process for clinical research. Many now have query capabilities that enable research teams to efficiently identify potentially eligible patients by searching structured data fields for specific eligibility criteria []. This automation has reduced the need for developing custom patient questionnaires to screen patients for eligibility. Artificial intelligence applied to EHR records can also now screen patients for eligibility criteria [,]. In addition, EHR systems can now directly engage potential participants through patient portals, automatically notifying them of trials that match their clinical profile. Although only a small percentage of patients contacted this way enroll as participants, this approach is effective at increasing recruitment because of the large volume of patients contacted through patient portal messages [-]. Integrating this EHR-based recruitment method with the patient attribute question can create a synergistic approach to optimize patient enrollment. Using multiple recruitment methods can increase enrollment yield [,].
Although this study was conducted at a single institution, the proposed approach is readily adaptable to other health systems that routinely collect patient-reported data during clinical visits. As technology advances, patient-reported data collection is becoming increasingly commonplace []. Adding a single question that requires no score calculations to existing questionnaire sets is straightforward and minimally increases the burden of patient response.
Several important limitations should be noted. First, although participants in the cognitive interviews reported a wide range of health conditions, they had more education than the general population, and approximately one-third of the interviewees (11/32, 34%) had previously participated in a clinical research study. Their opinions may differ from those with different educational backgrounds and experience with clinical research. Second, our assessment focused solely on the relationship between “research perception” questions and interest in a precision medicine registry. Patient attitudes may vary substantially across different types of clinical research. Third, responses to research participation questions might differ significantly if patients were facing an actual serious medical diagnosis rather than a hypothetical scenario. Fourth, our methodology for maintaining screening logs limited our ability to track actual study participation in the precision medicine registry. Instead, we could only measure the relationship between “research perception” questions and patients’ initial interest in learning more about the study. Finally, relying on patient-entered questionnaires as a screening tool has inherent limitations. This approach would only capture data from patients who complete electronic questionnaires, potentially underrepresenting racial and ethnic minority groups or populations considered vulnerable who may have limited access to patient portals [].
The ability to identify patients who have a greater likelihood of participating in research studies by assessing patients’ attitudes toward research is a helpful tool to aid patient recruitment into clinical research. This approach allows research teams to prioritize their recruitment efforts more effectively without relying on strict eligibility criteria. It provides a framework for more nuanced and respectful engagement in the recruitment process, ultimately serving both research efficiency and patient experience. The use of broad indicators rather than specific eligibility questions creates a more flexible and sustainable recruitment strategy that respects patient preferences while maximizing research participation opportunities. Further evaluation of this approach is warranted to assess the ability to use patient-reported questions on attitudes toward research for other types of clinical trials that vary by risk of adverse outcomes, level of patient burden, and health condition.
This work was supported by the Cleveland Clinic Center for Clinical Genomics.
The datasets generated and analyzed during this study are available from the corresponding author on reasonable request.
None declared.
Edited by A Stone, A Mavragani; submitted 19.May.2025; peer-reviewed by A Debbarma, R Omachonu; comments to author 07.Aug.2025; revised version received 28.Aug.2025; accepted 23.Sep.2025; published 29.Oct.2025.
©Irene Katzan, WH Wilson Tang, Andrew Schuster, Ryan Honomichl, Misti Allison, Renee Feldman, Michelle Gandolf, Benjamin Walter, Brittany Lapin. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 29.Oct.2025.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research (ISSN 1438-8871), is properly cited. The complete bibliographic information, a link to the original publication on https://www.jmir.org/, as well as this copyright and license information must be included.

As Vogue descended upon Tinsel Town for the fourth annual installment of Vogue World—this year in support of the Entertainment Community Fund—Vogue100 gathered for an intimate evening honoring Women in Film. Founded in 1973, the organization…

Angers Ducs (FRA), SG Cortina (ITA), Gyergyoi HK (ROU), and HK Mogo (LAT) are set for the 2026 IIHF Continental Cup, Round 2, Group C.
The penultimate stage of the pan-European club competition will be held at Angers IceParc…

AbbVie Announces Positive Topline Results from Phase 3 Pivotal Studies Evaluating Upadacitinib (RINVOQ®) in Adults and Adolescents with Vitiligo
NORTH CHICAGO, Ill., Oct. 29, 2025 /PRNewswire/ — AbbVie (NYSE: ABBV) today announced positive topline results from two replicate Phase 3 studies evaluating the safety and efficacy of upadacitinib (RINVOQ®;15 mg, once daily in adult and adolescent patients living with non-segmental vitiligo (NSV).1 NSV, the most common form of vitiligo (occurring in over 90% of patients), is characterized by symmetrical and bilateral white patches on both sides of the body.2-4
“Vitiligo is more than a skin condition – it’s a chronic autoimmune disease that can deeply affect a person’s confidence, identity and daily life,” said Kori Wallace, M.D., Ph.D., vice president, global head of immunology clinical development, AbbVie. “There are no approved systemic medical therapies for achieving re-pigmentation in vitiligo. These Phase 3 results represent a significant milestone in AbbVie’s commitment to supporting patients and expanding our immunology portfolio to deliver innovative solutions.”
The Total Vitiligo Area Scoring Index (T-VASI) is a tool that measures the extent of de-pigmentation over the entire body while F-VASI measures de-pigmentation of the face, an area among the most visible and psychosocially impactful for people living with NSV. Across both studies approximately 70% of subjects had T-VASI > 10 at baseline. In both studies, upadacitinib achieved the co-primary endpoints of T-VASI 50 (≥50% reduction from baseline in T-VASI score) and F-VASI 75 (≥75% reduction from baseline in F-VASI score) at week 48 versus placebo. Across both studies, statistically significant differences were observed with upadacitinib versus placebo in key ranked secondary endpoints, including F-VASI 50 at week 48.1
Key efficacy results are summarized below:
|
Phase 3 Efficacy Results1 |
||||
|
Study 1 |
Study 2 |
|||
|
Upadacitinib 15 mg (N=206), % |
Placebo (N=102), % |
Upadacitinib 15 mg (N=205), % |
Placebo (N=101), % |
|
|
Co-Primary Endpoints |
||||
|
T-VASI 50 at week 48 |
19.4 |
5.9 |
21.5 |
5.9 |
|
F-VASI 75 at week 48 |
25.2 |
5.9 |
23.4 |
6.9 |
|
Secondary Endpoints |
||||
|
F-VASI 50 at week 48 |
48.1 |
12.7 |
43.4 |
12.9 |
“For many people living with vitiligo, the journey is marked by uncertainty, frustration and a lack of medicines that can treat the disease systemically,” said Thierry Passeron, M.D., Ph.D., professor and chair, Department of Dermatology, Université Côte d’Azur. “These positive results indicate that targeting the underlying inflammation may offer a systemic treatment option which could help patients achieve visible results.”
The safety profile of upadacitinib in both studies was generally consistent with that observed in approved indications. No new safety signals were observed. Across both studies, the most frequent treatment-emergent adverse events (TEAEs) in the 48 weeks upadacitinib treatment groups were upper respiratory tract infection, acne and nasopharyngitis. In study 1, treatment-emergent serious adverse events (TESAEs) occurred in 3.9% and 4% of patients in the upadacitinib 15 mg and placebo groups, respectively. In study 2, TESAEs occurred in 2% and 1% of patients in the upadacitinib 15 mg and placebo groups, respectively. There were no adjudicated cases of any major cardiovascular event (MACE) or venous thromboembolism (VTE) in the studies. Three malignancy events were reported: one was reported in both placebo groups (1% each) of studies 1 and 2, and one was reported in the upadacitinib 15 mg group (0.5%) in study 1 (genital neoplasm). There were no deaths reported in the upadacitinib treated groups across both studies. There was one death reported in the placebo group in study 2.1
Use of upadacitinib in NSV is not approved and its safety and efficacy have not been evaluated by regulatory authorities.
About Vitiligo
Vitiligo is a chronic autoimmune disease characterized by the loss of pigment-producing cells (melanocytes), resulting in white patches of skin that can appear anywhere on the body and at any time. It is the most common depigmenting disorder worldwide, affecting approximately 0.5% to 2.3% of the global population. Non-segmental vitiligo (NSV) is the predominant form of the disease, accounting for roughly 84% of cases. NSV typically presents as symmetrical, bilateral patches on both sides of the body. While location varies, many patients report patches on critical areas such as the face, feet, hands and groin. The daily challenges of living with vitiligo can lead to mental health conditions, with a high prevalence of depression and anxiety.
About Viti-Up Clinical Trials
Upadacitinib M19-044 was conducted under a single protocol encompassing two replicate Phase 3 studies (Study 1 and Study 2) with independent randomization, investigative sites, data collection, analysis and reporting for each study. The trials were designed to evaluate the efficacy, safety and tolerability of upadacitinib in adult and adolescent patients (ages 12 and older) living with non-segmental vitiligo (NSV) who were eligible for systemic therapy. In Period A of both studies, participants were randomized in a 2:1 ratio to receive either upadacitinib 15 mg once daily or placebo for 48 weeks. Participants who completed Period A were eligible to enter Period B, a 112-week open-label extension in which all patients received upadacitinib 15 mg once daily. In total, Study 1 and Study 2 Periods A and B span 160 weeks. The two trials randomized 614 participants with NSV across 90 sites worldwide. More information on these trials can be found at www.clinicaltrials.gov (NCT06118411).
The co-primary endpoints were based on the achievement of Total Vitiligo Area Scoring Index (T-VASI) 50, defined as at least 50% reduction in T-VASI from baseline, at week 48, and the achievement of Facial Vitiligo Area Scoring Index (F-VASI) 75, defined as at least 75% reduction in F-VASI from baseline, at week 48 with the treatment of upadacitinib 15 mg compared with placebo in adults and adolescents with NSV.
The secondary endpoints include the achievement of F-VASI 50, defined as at least a 50% reduction in F-VASI from baseline, at week 48, and the achievement of F-VASI 75, defined as at least a 75% reduction in facial vitiligo area from baseline, at week 24. These endpoints were designed to assess the degree and timing of re-pigmentation on the face, an area among the most visible and psychosocially impactful for people living with NSV. Study 1 also included an analysis using 3D digital imaging to assess facial repigmentation, measured in a subset of the study population.
About RINVOQ® (upadacitinib)
Discovered and developed by AbbVie scientists, RINVOQ is a JAK inhibitor that is being studied in several immune-mediated inflammatory diseases. In human leukocyte cellular assays, RINVOQ inhibited cytokine-induced STAT phosphorylation mediated by JAK1 and JAK1/JAK3 more potently than JAK2/JAK2 mediated STAT phosphorylation. The relevance of inhibition of specific JAK enzymes to therapeutic effectiveness and safety is not currently known.
Upadacitinib (RINVOQ) is being studied in Phase 3 clinical trials for alopecia areata, hidradenitis suppurativa, Takayasu arteritis, systemic lupus erythematosus and vitiligo.
RINVOQ (upadacitinib) U.S. Uses and Important Safety Information5
RINVOQ is a prescription medicine used to treat:
It is not known if RINVOQ is safe and effective in children with ankylosing spondylitis, non-radiographic axial spondyloarthritis, ulcerative colitis, or Crohn’s disease.
It is not known if RINVOQ is safe and effective in children under 12 years of age with atopic dermatitis.
It is not known if RINVOQ LQ is safe and effective in children with atopic dermatitis.
RINVOQ/RINVOQ LQ is a prescription medicine used to treat:
It is not known if RINVOQ/RINVOQ LQ is safe and effective in children under 2 years of age with polyarticular juvenile idiopathic arthritis or psoriatic arthritis.
IMPORTANT SAFETY INFORMATION FOR RINVOQ/RINVOQ LQ (upadacitinib)
What is the most important information I should know about RINVOQ*?
RINVOQ may cause serious side effects, including:
Do not take RINVOQ if you are allergic to upadacitinib or any of the ingredients in RINVOQ. See the Medication Guide or Consumer Brief Summary for a complete list of ingredients.
What should I tell my HCP BEFORE starting RINVOQ?
Tell your HCP if you:
|
̶ Fever, sweating, or chills ̶ Shortness of breath ̶ Warm, red, or painful skin or sores on your body |
̶ Muscle aches ̶ Feeling tired ̶ Blood in phlegm ̶ Diarrhea or stomach pain |
̶ Cough ̶ Weight loss ̶ Burning when urinating or urinating more often than normal |
Tell your HCP about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. RINVOQ and other medicines may affect each other, causing side effects.
Especially tell your HCP if you take:
If you are not sure if you are taking any of these medicines, ask your HCP or pharmacist.
What should I avoid while taking RINVOQ?
Avoid food or drink containing grapefruit during treatment with RINVOQ as it may increase the risk of side effects.
What should I do or tell my HCP AFTER starting RINVOQ?
|
̶ Swelling ̶ Pain or tenderness in one or both legs |
̶ Sudden unexplained chest or upper back pain ̶ Shortness of breath or difficulty breathing |
What are other possible side effects of RINVOQ?
Common side effects include upper respiratory tract infections (common cold, sinus infections), shingles (herpes zoster), herpes simplex virus infections (including cold sores), bronchitis, nausea, cough, fever, acne, headache, swelling of the feet and hands (peripheral edema), increased blood levels of creatine phosphokinase, allergic reactions, inflammation of hair follicles, stomach-area (abdominal) pain, increased weight, flu, tiredness, lower number of certain types of white blood cells (neutropenia, lymphopenia, leukopenia), muscle pain, flu-like illness, rash, increased blood cholesterol levels, increased liver enzyme levels, pneumonia, low number of red blood cells (anemia), and infection of the stomach and intestine (gastroenteritis).
A separation or tear to the lining of the back part of the eye (retinal detachment) has happened in people with atopic dermatitis treated with RINVOQ. Call your HCP right away if you have any sudden changes in your vision during treatment with RINVOQ.
Some people taking RINVOQ may see medicine residue (a whole tablet or tablet pieces) in their stool. If this happens, call your HCP.
These are not all the possible side effects of RINVOQ.
How should I take RINVOQ/RINVOQ LQ?
RINVOQ is taken once a day with or without food. Do not split, crush, or chew the tablet. Take RINVOQ exactly as your HCP tells you to use it. RINVOQ is available in 15 mg, 30 mg, and 45 mg extended-release tablets. RINVOQ LQ is taken twice a day with or without food. RINVOQ LQ is available in a 1 mg/mL oral solution. RINVOQ LQ is not the same as RINVOQ tablets. Do not switch between RINVOQ LQ and RINVOQ tablets unless the change has been made by your HCP.
*Unless otherwise stated, “RINVOQ” in the IMPORTANT SAFETY INFORMATION refers to RINVOQ and RINVOQ LQ.
This is the most important information to know about RINVOQ. For more information, talk to your HCP.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
If you are having difficulty paying for your medicine, AbbVie may be able to help. Visit AbbVie.com/PatientAccessSupport to learn more.
Please click here for the Full Prescribing Information and Medication Guide.
Globally, prescribing information varies; refer to the individual country product label for complete information.
About AbbVie
AbbVie’s mission is to discover and deliver innovative medicines and solutions that solve serious health issues today and address the medical challenges of tomorrow. We strive to have a remarkable impact on people’s lives across several key therapeutic areas including immunology, oncology, neuroscience and eye care – and products and services in our Allergan Aesthetics portfolio. For more information about AbbVie, please visit us at www.abbvie.com. Follow @abbvie on LinkedIn, Facebook, Instagram, X (formerly Twitter) and YouTube.
Forward-Looking Statements
Some statements in this news release are, or may be considered, forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995. The words “believe,” “expect,” “anticipate,” “project” and similar expressions and uses of future or conditional verbs, generally identify forward-looking statements. AbbVie cautions that these forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those expressed or implied in the forward-looking statements. Such risks and uncertainties include, but are not limited to, challenges to intellectual property, competition from other products, difficulties inherent in the research and development process, adverse litigation or government action, changes to laws and regulations applicable to our industry, the impact of global macroeconomic factors, such as economic downturns or uncertainty, international conflict, trade disputes and tariffs, and other uncertainties and risks associated with global business operations. Additional information about the economic, competitive, governmental, technological and other factors that may affect AbbVie’s operations is set forth in Item 1A, “Risk Factors,” of AbbVie’s 2024 Annual Report on Form 10-K, which has been filed with the Securities and Exchange Commission, as updated by its Quarterly Reports on Form 10-Q and in other documents that AbbVie subsequently files with the Securities and Exchange Commission that update, supplement or supersede such information. AbbVie undertakes no obligation, and specifically declines, to release publicly any revisions to forward-looking statements as a result of subsequent events or developments, except as required by law.
References:
Contacts:
SOURCE AbbVie
Kikuchi K, Hamaguchi S. Novel sex-determining genes in fish and sex chromosome evolution. Dev Dyn. 2013;242(4):339–53.
Google Scholar
Kang Y, Guan GJ, Hong YH. Insights of sex…
With populations aging rapidly worldwide, heart failure has become one of the most pressing medical and social challenges. Older patients face not only a high mortality risk but also losses in muscle strength, mobility, and…