Harry Maguire’s late header secured Manchester United’s first win at Anfield in almost a decade and gave head coach Ruben Amorim back-to-back league victories for the first time in his tenure.
The visitors looked to have been denied by Cody…

Harry Maguire’s late header secured Manchester United’s first win at Anfield in almost a decade and gave head coach Ruben Amorim back-to-back league victories for the first time in his tenure.
The visitors looked to have been denied by Cody…

For two years, Ismail Baba had dreamed of a ceasefire. The father of four had been displaced 11 times during the war in Gaza and was struggling to find adequate food for his children amid a worsening hunger crisis.
When a ceasefire was announced…
![Body Horror Zoo Sim Game ‘Zoochosis’ Finally Heads to Xbox on October 24 [Trailer]](https://afnnews.qaasid.com/wp-content/uploads/2025/10/zoochosis.jpg)
Clapperheads’ body horror zoo sim Zoochosis will finally be heading to the Xbox. After last year’s Steam launch, and the PlayStation and Epic Games Store launch this past August, the game will arrive on the Xbox Series and Xbox…
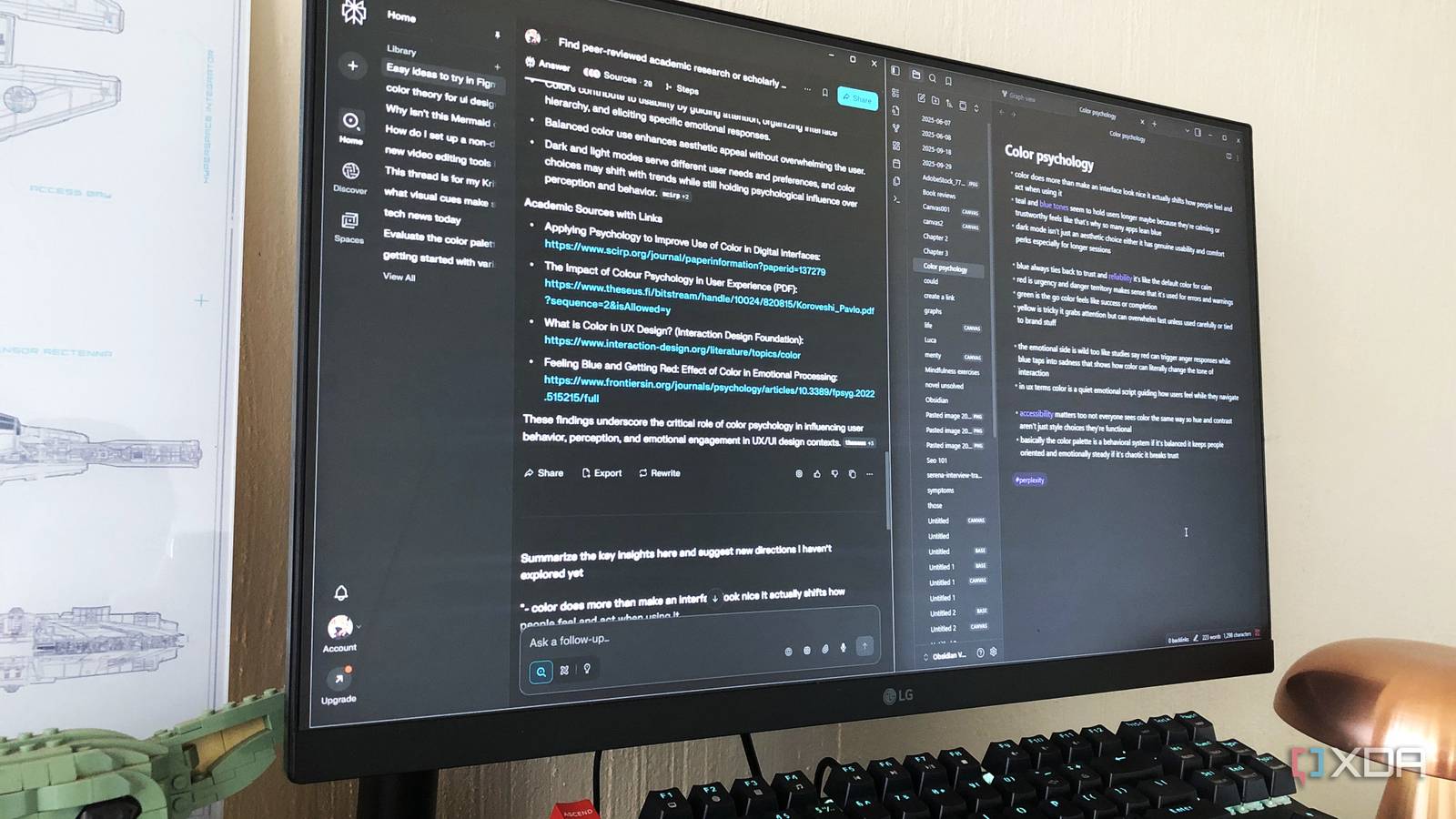
One of the Obsidian plugins I can’t live without is QuickAdd. It helps me save time by reducing the clicks. The plugin also helps me keep my notes consistent from templates by adding dates, titles, and auto-filled fields. It’s a free Obsidian…

In the phase 3 DREAM3R trial (NCT04334759, ACTRN12620001199909), first-line durvalumab (Imfinzi) plus chemotherapy demonstrated consistent overall survival (OS), progression-free survival (PFS), and objective response rates (ORR)benefits in advanced pleural mesothelioma as in previous phase 2 trials. However, slow accrual and standard-of-care practice changes created inconclusive results and leave unanswered questions.1
Data were presented at the 2025 European Society for Medical Oncology (ESMO) Congress. Durvalumab and chemotherapy yielded a median OS of 21 months (95% CI, 18–27) vs 18 months for chemotherapy alone (95% CI, 14–30) for a HR of 0.92 (95% CI, 0.63–1.36; stratified log-rank P =.9). Median PFS were 8 months (95% CI, 8–10) with the combination vs 7 months (95% CI, 6–8) with chemotherapy alone (HR, 0.70; 95% CI, 0.5–0.98; P =.20). ORRs were 58% and 35%, respectively (P =.005).
The safety profiles of chemotherapy with or without durvalumab were consistent with the known profiles of the agents. Fatigue, nausea, and anemia were the most common adverse events (AEs) in both arms.
Durvalumab was assessed in the phase 2 DREAM (ACTRN12616001170415) and PrE0505 (NCT02899195). In these studies, durvalumab showed promising activity in combination with pemetrexed and either cisplatin or carboplatin chemotherapy.
The phase 3 DREAM3R study randomized patients 2:1 to receive durvalumab 1500 mg every 3 weeks plus chemotherapy every 3 weeks for 4 to 6 cycles, followed by durvalumab maintenance 1500 mg every 4 weeks until progressive disease or unacceptable toxicity, or chemotherapy every 3 weeks for 4 to 6 cycles. Accrual started in February 2021.
However, the results of the CheckMate 743 study (NCT02899299) published in 2021 showed that nivolumab (Opdivo) and ipilimumab (Yervoy) significantly improved OS vs chemotherapy.2 As the standard of care in pleural mesothelioma had changed, the study design changed to incorporate a nivolumab/ipilimumab arm, and randomization changed to 1:1 durvalumab/chemotherapy or physician’s choice of nivolumab/ipilimumab or chemotherapy.1 At this ESMO Congress, only data from the durvalumab and chemotherapy cohorts were presented.
A total of 114 patients received chemotherapy and durvalumab and 60 patients received chemotherapy alone. The study’s primary end point was OS. Secondary end points included PFS, ORR, and AEs.
During her presentation, Anna Nowak, MD, deputy vice chancellor at the University of Western Australia, noted that, while DREAM3R was a well-designed study, due to the early stopping of DREAM3R, it is unlikely that the study question will ever be answered.
“The key limitation is that slow accrual led to early stopping, and this slow accrual was no doubt, an artifact, partly of the global pandemic, but also then of a change of clinical practice as a result of the of the CheckMate 743 study and subsequent regulatory approval and standard clinical care use,” Nowak explained. “These design modifications add complexity to the interpretation of this clinical trial, and of course, chemotherapy alone would no longer be considered standard of care.”
Nowak did note that the high objective tumor response and control observed with chemotherapy and durvalumab raised the potential for future testing in the neoadjuvant setting. Additionally, correlative biomarkers that were collected from DREAM3R are also under ongoing analysis.
Further, Nowak emphasized the importance of timely activation of randomized phase 3 trials after positive phase 2 trials “is critically important to seize the window of opportunity for accrual and provide reliable answers.” Nowak added that, “the reasons for the delay in starting this trial were multifactorial.”
DISCLOSURES: Nowak declared participation on AstraZeneca Data Safety Monitoring Board, board membership on Cancer Council Western Australia, position on Cancer Australia Advisory Council, and president-elect of the International Mesothelioma Interest Group.
As Streptococcus pneumoniae (S. pneumoniae)bacteria evolves over time, vaccine development is forced to follow suit in order to keep patients protected against pneumococcal diseases and their shifting serotypes, according to a session presented…
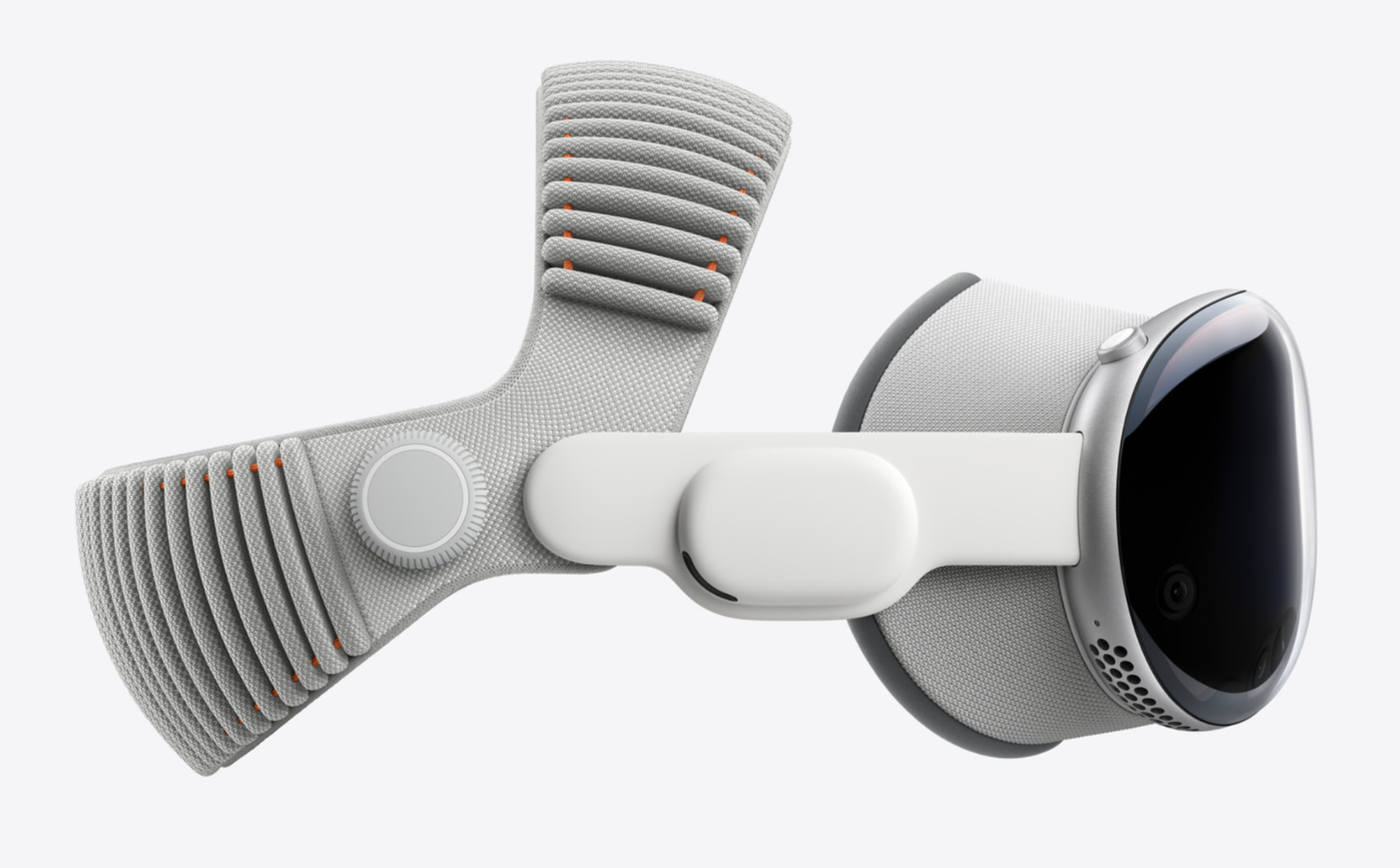
Today’s Above Avalon Daily update includes the following stories:
Happy Wednesday. It’s been a busy day with Apple unveiling updates to products across three different categories (iPad, Mac, and Vision). Today’s update will examine the M5…