Pakistan links Afghan trade resumption to border security, notes progress in Doha talks
ISLAMABAD: Pakistan on Friday ruled out the resumption of bilateral and transit trade…

ISLAMABAD: Pakistan on Friday ruled out the resumption of bilateral and transit trade…

As a predominant chronic pulmonary condition in pediatric populations, bronchial asthma is characterized by sustained inflammatory processes in the bronchial tubes, airflow obstruction that is physiologically reversible, and…
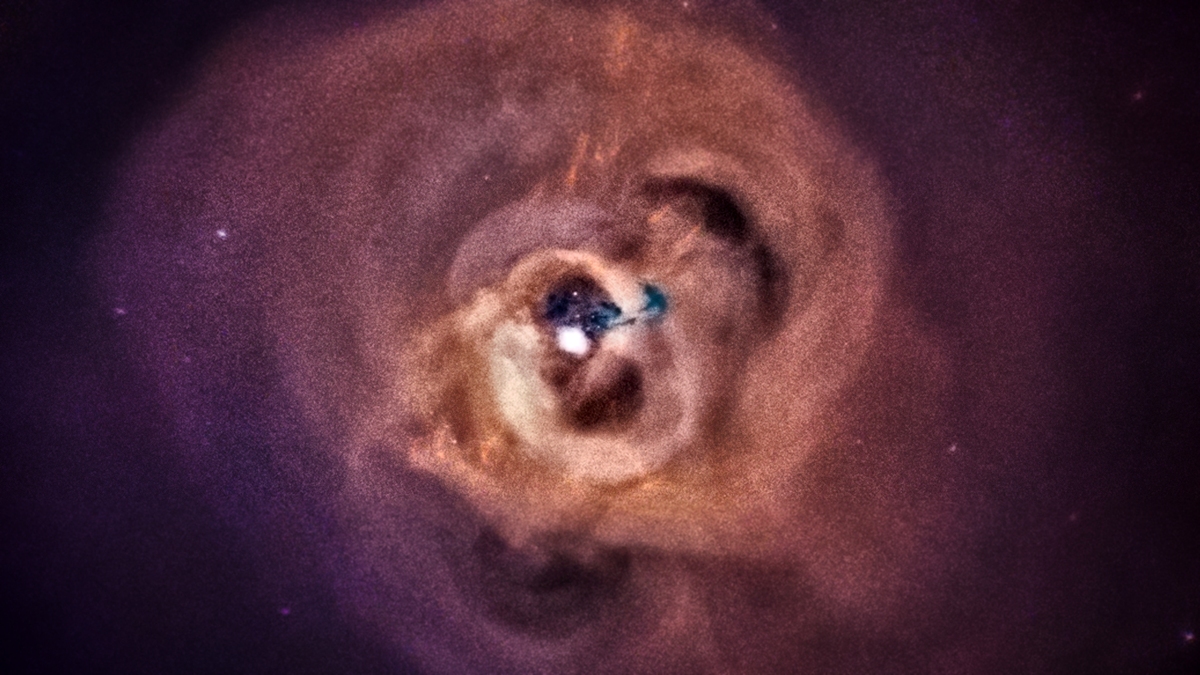
NASA created a haunting audio clip of sound waves rippling out of a supermassive black hole, located 250 million light-years away.
The black hole is at the center of the Perseus cluster of galaxies, and the acoustic waves coming from it have…
Danvers, MA – Oct 24, 2025 – Johnson & Johnson MedTech, a global leader in heart recovery, will feature the latest long-term data confirming the survival benefit of Impella CP and present the newest Impella…
SPRING HOUSE, Pa. (October 24, 2025) – Johnson & Johnson (NYSE: JNJ) today announced new long-term 52-week data from the Phase 3 ICONIC-TOTAL studya evaluating icotrokinra, a first-in-class investigational targeted oral peptide that precisely blocks the IL-23 receptor, in adults and pediatric patients 12 years of age and older (adolescents) with plaque psoriasis (PsO) affecting high-impact sites.
The ICONIC-TOTAL study, presented at the 2025 Fall Clinical Dermatology Conference, simultaneously evaluated adults and adolescents with at least moderate scalp, genital and/or hand/foot plaque psoriasis with ≥1% Body Surface Area (BSA) affected. Through Week 52, icotrokinra demonstrated high and durable rates of site-specific psoriasis clearance affecting all of these high-impact and difficult-to-treat areas of the body.1
In the smaller subset of patients with hand/foot psoriasis, treatment with icotrokinra showed a numerically higher rate of skin clearance at Week 16, which increased through Week 52 with patients achieving a hand and/or foot Physician’s Global Assessment (hf-PGA)e score of 0/1 increasing from 42% to 62%.
“Many of the patients in my practice experience significant distress when psoriasis affects sensitive areas such as the scalp, genitals, hands, and feet,” said Edward (Ted) Lain, MD, MBA Executive Director of the Austin Institute for Clinical Research in Austin, Texas, and study investigator. “The durable response rates observed in the ICONIC-TOTAL study show that icotrokinra has the potential to be a meaningful new option for effectively managing moderate-to-severe plaque psoriasis long-term in both adults and adolescents.”
Overall response rates among patients treated with once daily icotrokinra were maintained through Week 52, with 67% of patients treated with icotrokinra achieving clear or almost clear skin (Investigator’s Global Assessment (IGA)f 0/1) and 44% achieving completely clear skin (IGA 0) at Week 52. The overall response rates were also comparable among patients who received icotrokinra for all 52 weeks and those who transitioned from placebo to icotrokinra at Week 16 (67% versus 68% achieved IGA 0/1, respectively). Across treatment groups, adverse event and serious adverse event rates were similar through Week 52 compared to those through Week 16, with no new safety signals identified.
“The new long-term data from ICONIC-TOTAL adds to the robust findings seen across several studies this year, including the recently reported ICONIC-LEAD 52-week data,” said Liza O’Dowd, MD, Vice President, Immunodermatology and Respiratory Disease Areas Lead, Johnson & Johnson Innovative Medicine. “Psoriasis that affects high-impact skin sites often results in greater physical discomfort for patients due to the sensitivity of these areas. Icotrokinra is being developed with the goal of setting a new standard of treatment that offers patients the precision of a targeted therapy, high level skin clearance and favorable safety profile with the ease of a once daily pill.”
Editor’s notes:
a. ICONIC-TOTAL evaluates the efficacy and safety of icotrokinra compared with placebo in participants with at least moderate scalp, genital, and/or hand/foot PsO with once daily icotrokinra or placebo, with placebo-to-icotrokinra transition at Week 16.
b. The ss-IGA is a five-point scale where scalp lesions are assessed in terms of clinical signs of redness, thickness, and scaliness on a severity score ranging from 0 to 4, where 0 indicates absence of disease, 1 is very mild, 2 is mild, 3 is moderate and 4 indicates severe disease.
c. The sPGA-G is a six-point scale used to evaluate the severity of genital psoriasis at a given time point ranging from 0 to 5, where 0 indicates clear, 1 is minimal, 2 is mild, 3 is moderate, 4 is severe and 5 indicates very severe disease.2
d. The Physician’s Global Assessment of Psoriasis on the Hands and/or Feet (hf-PGA) assesses the severity of hand and foot psoriasis using a 5-point scale to score the plaques on the hands and feet as: clear (0), almost clear (1), mild (2), moderate (3) and severe (4).3
e. Dr. Lain is a paid consultant for Johnson & Johnson. He has not been compensated for any media work.
f. The IGA is a five-point scale with a severity score ranging from 0 to 4, where 0 indicates clear, 1 is minimal, 2 is mild, 3 is moderate, and 4 indicates severe disease.4
About the ICONIC Clinical Development Program
The pivotal Phase 3 ICONIC clinical development program of icotrokinra (JNJ-2113) in adult and pediatric patients 12 years of age and older with moderate-to-severe plaque PsO was initiated with two studies in Q4 2023 – ICONIC-LEAD and ICONIC-TOTAL – pursuant to the license and collaboration agreement between Protagonist Therapeutics, Inc. and Janssen Biotech, Inc., a Johnson & Johnson company.5
ICONIC-LEAD (
NCT06095115) is a RCT to evaluate the efficacy and safety of icotrokinra compared with placebo in participants with moderate-to-severe plaque PsO, with PASI 90 and IGA score of 0 or 1 with at least a 2-grade improvement as co-primary endpoints.6
ICONIC-TOTAL (
NCT06095102) is a RCT to evaluate the efficacy and safety of icotrokinra compared with placebo for the treatment of PsO in participants with at least moderate severity affecting special areas (e.g., scalp, genital, and/or hands and feet) with overall IGA score of 0 or 1 with at least a 2-grade improvement as the primary endpoint.7
Other Phase 3 studies in the development program include ICONIC-ADVANCE 1 (
NCT06143878) and ICONIC-ADVANCE 2 (
NCT06220604), which are evaluating the efficacy and safety of icotrokinra compared with both placebo and deucravacitinib in adults with moderate-to-severe plaque PsO.8,9 ICONIC-ASCEND will evaluate the efficacy and safety of icotrokinra compared with placebo and ustekinumab in participants with moderate-to-severe plaque psoriasis. ICONIC-PsA 1 (
NCT06878404) and ICONIC-PsA 2 (
NCT06807424) will evaluate the efficacy and safety of icotrokinra compared to placebo in participants with active psoriatic arthritis.10,11 ICONIC-UC (
NCT07196748) will evaluate the efficacy and safety of icotrokinra in adults and adolescents with moderately to severely active ulcerative colitis (UC) and ICONIC-CD (
NCT07196722) will evaluation the efficacy and safety of icotrokinra in adults with moderately to severely active Crohn’s disease (CD).12,13
About Plaque Psoriasis
Plaque psoriasis (PsO) is a chronic immune-mediated disease resulting in overproduction of skin cells, which causes inflamed, scaly plaques that may be itchy or painful.14 It is estimated that 8 million Americans and more than 125 million people worldwide live with the disease.15 Nearly one-quarter of all people with plaque PsO have cases that are considered moderate-to-severe.15 Plaques typically appear as raised patches with a silvery white buildup of dead skin cells or scales. Plaques may appear red in lighter skin or more of a purple, gray or dark brown color in patients with darker skin tones. Plaques can appear anywhere on the body, although they most often appear on the scalp, knees, elbows, and torso.16 Living with plaque PsO can be a challenge and impact life beyond a person’s physical health, including emotional health, relationships, and handling the stressors of life.17 Psoriasis on highly visible areas of the body or sensitive skin, such as the scalp, hands, feet, and genitals, can have an increased negative impact on quality of life.16,18
About Icotrokinra (JNJ-77242113, JNJ-2113)
Investigational icotrokinra is the first targeted oral peptide designed to precisely block the IL-23 receptor,19 which underpins the inflammatory response in moderate-to-severe plaque PsO, ulcerative colitis and offers potential in other IL-23-mediated diseases.20,21 Icotrokinra binds to the IL-23 receptor with single-digit picomolar affinity and demonstrated potent, precise inhibition of IL-23 signaling in human T cells.22 The license and collaboration agreement established between Protagonist Therapeutics, Inc. and Janssen Biotech, Inc., a Johnson & Johnson company, in 2017 enabled the companies to work together to discover and develop next-generation compounds that ultimately led to icotrokinra.23
Icotrokinra was jointly discovered and is being developed pursuant to the license and collaboration agreement between Protagonist and Johnson & Johnson. Johnson & Johnson retains exclusive worldwide rights to develop icotrokinra in Phase 2 clinical trials and beyond, and to commercialize compounds derived from the research conducted pursuant to the agreement against a broad range of indications.24,25,26
Icotrokinra is being studied in the pivotal Phase 3 ICONIC clinical development program in moderate-to-severe plaque psoriasis, active psoriatic arthritis, moderately to severely active ulcerative colitis and moderately to severely active Crohn’s disease.
About Johnson & Johnson
At Johnson & Johnson, we believe health is everything. Our strength in healthcare innovation empowers us to build a world where complex diseases are prevented, treated, and cured, where treatments are smarter and less invasive, and solutions are personal. Through our expertise in Innovative Medicine and MedTech, we are uniquely positioned to innovate across the full spectrum of healthcare solutions today to deliver the breakthroughs of tomorrow and profoundly impact health for humanity.
Learn more at
https://www.jnj.com/ or at
www.innovativemedicine.jnj.com. Follow us at @JNJInnovMed.
Janssen Biotech, Inc. is a Johnson & Johnson company.
Cautions Concerning Forward-Looking Statements
This press release contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding icotrokinra (JNJ-2113). The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson. Risks and uncertainties include, but are not limited to: challenges and uncertainties inherent in product research and development, including the uncertainty of clinical success and of obtaining regulatory approvals; uncertainty of commercial success; manufacturing difficulties and delays; competition, including technological advances, new products and patents attained by competitors; challenges to patents; product efficacy or safety concerns resulting in product recalls or regulatory action; changes in behavior and spending patterns of purchasers of health care products and services; changes to applicable laws and regulations, including global health care reforms; and trends toward health care cost containment. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson’s most recent Annual Report on Form 10-K, including in the sections captioned “Cautionary Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” and in Johnson & Johnson’s subsequent Quarterly Reports on Form 10-Q and other filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. Johnson & Johnson does not undertake to update any forward-looking statement as a result of new information or future events or developments.
Footnotes
1 Lain, E et al. Durability of Response to the Targeted Oral Peptide Icotrokinra for High-Impact Site Psoriasis: 1-Year ICONIC-TOTAL Findings. Oral presentation (Presentation FC01.1G) at the 2025 Fall Clinical Dermatology Conference. October 2025.
2 Merola JF, Bleakman AP, Gottlieb AB, et al. The Static Physician’s Global Assessment of Genitalia: a clinical outcome measure for the severity of genital psoriasis. J Drugs Dermatol. 2017;16(8):793-799
3 Goldblum O, et al. Validation of the physician’s global assessment of psoriasis of the hands and/or feet as a clinical endpoint. J Am Acad Dermatol. 2013:68(4)Supplement1:AB218.
4 Simpson E, Bissonnette R, Eichenfield LF, et al. The validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD™): The development and reliability testing of a novel clinical outcome measurement instrument for the severity of atopic dermatitis [published online April 25, 2020]. J Am Acad Dermatol. doi: 10.1016/j.jaad.2020.04.104. Accessed April 2025.
5 Protagonist Therapeutics. Press release. Protagonist announces advancement of JNJ-2113 across multiple indications. Available at: https://www.accesswire.com/791174/protagonist-announces-advancement-of-jnj-2113-across-multiple-indications. Accessed August 2025.
6 Clinicaltrials.gov. A study of JNJ-2113 in adolescent and adult participants with moderate-to-severe plaque psoriasis (ICONIC-LEAD). Identifier NCT06095115. https://classic.clinicaltrials.gov/ct2/show/NCT06095115. Accessed July 2025.
7 Clinicaltrials.gov. A study of JNJ-2113 for the treatment of participants with plaque psoriasis involving special areas (scalp, genital, and/or palms of the hands and the soles of the feet) (ICONIC-TOTAL). Identifier NCT06095102. https://classic.clinicaltrials.gov/ct2/show/NCT06095102. Accessed July 2025.
8 Clinicaltrials.gov. A Study of JNJ-77242113 for the Treatment of Participants With Moderate to Severe Plaque Psoriasis. Identifier NCT06143878. https://clinicaltrials.gov/study/NCT06143878. Accessed July 2025.
9 Clinicaltrials.gov. A Study of JNJ-77242113 for the Treatment of Participants With Moderate to Severe Plaque Psoriasis (ICONIC-ADVANCE 2). Identifier NCT06220604. https://clinicaltrials.gov/study/NCT06220604. Accessed July 2025.
10 Clinicaltrials.gov. A Study to Evaluate the Efficacy and Safety of JNJ-77242113 (Icotrokinra) in Biologic-naïve Participants With Active Psoriatic Arthritis (ICONIC-PsA 1). Identifier NCT06878404. https://clinicaltrials.gov/study/NCT06878404.
11 A Study to Evaluate the Efficacy and Safety of Icotrokinra (JNJ-77242113) in Biologic-experienced Participants With Active Psoriatic Arthritis (ICONIC-PsA 2). Identifier NCT06807424. https://clinicaltrials.gov/study/NCT06807424.
12 Clinicaltrials.gov. A Protocol of Icotrokinra Therapy in Adult and Adolescent Participants With Moderately to Severely Active Ulcerative Colitis (ICONIC-UC). Identifier NCT07196748. https://clinicaltrials.gov/study/NCT07196748. Accessed September 2025.
13 Clinicaltrials.gov. A Study of Icotrokinra in Participants With Moderately to Severely Active Crohn’s Disease. Identifier NCT07196722. https://clinicaltrials.gov/study/NCT07196722. Accessed October 2025.
14 National Psoriasis Foundation. About Psoriasis. Available at: https://www.psoriasis.org/about-psoriasis. Accessed July 2025.
15 National Psoriasis Foundation. Psoriasis Statistics. Available at: https://www.psoriasis.org/content/statistics. Accessed July 2025.
16 National Psoriasis Foundation. Plaque Psoriasis. Available at: https://www.psoriasis.org/plaque/. Accessed July 2025.
17 National Psoriasis Foundation. Life with Psoriasis. Available at: https://www.psoriasis.org/life-with-psoriasis/. Accessed July 2025.
18 National Psoriasis Foundation. High Impact Sites. Available at: https://www.psoriasis.org/high-impact-sites/. Accessed July 2025.
19 Bissonnette R, et al. Data presentation. A phase 2, randomized, placebo-controlled, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severe plaque psoriasis: FRONTIER 1. Presented at WCD 2023, July 3-8.
20 Razawy W, et al. The role of IL‐23 receptor signaling in inflammation‐mediated erosive autoimmune arthritis and bone remodeling. Eur J Immunol. 2018 Feb; 48(2): 220–229.
21 Tang C, et al. Interleukin-23: as a drug target for autoimmune inflammatory diseases. Immunology. 2012 Feb; 135(2): 112–124.
22 Pinter A, et al. Data Presentation. JNJ-77242113 Treatment Induces a Strong Systemic Pharmacodynamic Response Versus Placebo in Serum Samples of Patients with Plaque Psoriasis: Results from the Phase 2, FRONTIER 1 Study. Presented at EADV 2023, October 11-14.
23 Johnson & Johnson. Press release. Janssen enters into worldwide exclusive license and collaboration agreement with Protagonist Therapeutics, Inc. for the oral Interlukin-23 receptor antagonist drug candidate for the treatment of Inflammatory Bowel Disease. Available at: https://www.jnj.com/media-center/press-releases/janssen-enters-into-worldwide-exclusive-license-and-collaboration-agreement-with-protagonist-therapeutics-inc-for-the-oral-interlukin-23-receptor-antagonist-drug-candidate-for-the-treatment-of-inflammatory-bowel-disease. Accessed July 2025.
24 Protagonist Therapeutics. Press release. Protagonist Therapeutics announces amendment of agreement with Janssen Biotech for the continued development and commercialization of IL-23 antagonists. Available at: https://www.prnewswire.com/news-releases/protagonist-therapeutics-announces-amendment-of-agreement-with-janssen-biotech-for-the-continued-development-and-commercialization-of-il-23-antagonists-301343621.html. Accessed July 2025.
25 Protagonist Therapeutics. Press release. Protagonist Reports positive results from Phase 1 and pre-clinical studies of oral Interleukin-23 receptor antagonist JNJ-2113. Available at: https://www.prnewswire.com/news-releases/protagonist-reports-positive-results-from-phase-1-and-pre-clinical-studies-of-oral-interleukin-23-receptor-antagonist-jnj-2113-301823039.html. Accessed July 2025.
26 Protagonist Therapeutics. Press release. Protagonist Therapeutics announces positive topline results for Phase 2b FRONTIER 1 clinical trial of oral IL-23 receptor antagonist JNJ-2113 (PN-235) in psoriasis. Available at: https://www.prnewswire.com/news-releases/protagonist-therapeutics-announces-positive-topline-results-for-phase-2b-frontier-1-clinical-trial-of-oral-il-23-receptor-antagonist-jnj-2113-pn-235-in-psoriasis-301764181.html. Accessed July 2025.
The World Health Organization (WHO) would like to announce an upcoming Guideline Development Group meeting on the use of (1) a new class of technologies – near point-of-care (NPOC) molecular tests for the diagnosis of TB that differ…

The hour is upon us, and Insider is back again. As always, we’ve got the top headlines from international film and TV. Jesse Whittock on the buttons. Let’s begin, and sign up to the newsletter here.
Euro C-Suite Turbulence

QUICK FACTS
Name: Chestnut Ridge Falls, or “Eternal Flame Falls”
Location: Chestnut Ridge Park, Erie County, New York
Coordinates: 42.70158, -78.75113
Why it’s incredible: The falls house a natural gas seep that can burn uninterrupted.
Eternal Flame…

Welcome back to Haul of Fame, your must-read beauty roundup for new products, new ideas and cereal milk soft serve ice cream. Heaven.
Included in today’s issue: Avène, Bath & Body Works, Bioelements, Blank Beauty, Clean Reserve, ColourPop,…
Unlock the Editor’s Digest for free
Roula Khalaf, Editor of the FT, selects her favourite stories in this weekly newsletter.
All the answers here are linked in some way. Once you’ve spotted the connection, any you didn’t know the first time…