Formula 1’s 10 teams all successfully obliged with the championship cost cap in 2024, the FIA has confirmed, although Aston Martin has been noted for a minor “procedural breach.”
F1 introduced a cost cap in 2021 in a bid to create a more level…

Formula 1’s 10 teams all successfully obliged with the championship cost cap in 2024, the FIA has confirmed, although Aston Martin has been noted for a minor “procedural breach.”
F1 introduced a cost cap in 2021 in a bid to create a more level…

Bird flu cases are rising in the Northern Hemisphere, and outbreaks are likely to continue testing farmers and public health authorities determined to curb the spread this winter.
The current circulating “clade” of H5N1, which is mainly carried…

Saudi Arabia unveils plans for 46 000-seat Sky Stadium atop 350m tower in NEOM as part of World Cup 2034 bid
Saudi Arabia has revealed one of the most…

Palaeontologists have discovered a new species of ancient marine crocodile from Egypt’s Western Desert.
Based on the remains of four animals dug up in the arid plains of the Kharga Oasis, it is thought the species lived around 80 million years…
This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!

An emotional Katie Boulter retired from her first-round match at the Hong Kong Open with injury.
The Briton, 29, called for the trainer and was in tears after deciding she could not continue when trailing 20-year-old Filipino Alexandra Eala 6-4…
Updated data from the phase 1/2 OptimUM-01 trial (NCT03947385) shared during the
Investigators reported that the median OS was “notable compared to historical controls,” despite slightly more than one-third (39%) of patients having an ECOG performance status of 1. The median progression-free survival (PFS) with darovasertib plus crizotinib also compared favorably with historical controls. At a median follow-up of 25 months, the median PFS in the respective groups was 7.0 months (95% CI, 3.8-7.7) vs just 2.8 months (95% CI, 2.7-3.4).
The doublet (n = 41) elicited an objective response rate (ORR) of 34.1% (95% CI, 20.1%-50.6%), which was comprised entirely of partial responses; 56.1% of patients achieved stable disease, and 9.8% experienced disease progression. The median duration of response (DOR) was 9.0 months (95% CI, 3.8-12.0). The disease control rate achieved with the combination was 90.2% (95% CI, 76.9%-97.3%), with 85% of patients experiencing any reduction in target lesions.1,2
“These findings suggest that darovasertib plus crizotinib may represent a novel first-line treatment option and support the ongoing registrational phase 2/3 [OptimUM-02 trial (NCT05987332)] in the first-line setting,” Meredith McKean, MD, of Sarah Cannon Research Institute, in Nashville, Tennessee, and colleagues wrote in the presentation.1
It is known that those with metastatic uveal melanoma have a poor prognosis, which median PFS under 3 months and median OS ranging under 1 year. Moreover, the majority of these tumors are known to have PKC-activating mutations in GNAQ/11. Darovasertib targets PKC and has been shown to have activity in this disease. Preclinical evidence supports that crizotinib has complementary activity to darovasertib; as such, investigators sought to evaluate the combination in this population as part of the OptimUM-01 study.
The phase 1/2, multicenter, open-label trial enrolled patients with metastatic uveal melanoma with GNAQ/GNA11 mutations or PRKC fusions who were at least 18 years of age and had an ECOG performance status no higher than 1, measurable disease by RECIST 1.1 criteria, and acceptable organ function. Patients could not have had prior exposure to PKC/MET/GNAQ11 inhibitors in the metastatic setting, nor could they have symptomatic or untreated central nervous system metastases. They were allowed to have previously received ablations, had oligometastatic disease surgically resected, or received neoadjuvant or adjuvant therapy.
In the dose-expansion portion of the research, patients were administered darovasertib at a twice-daily dose of 300 mg and crizotinib at a twice-daily dose of 200 mg. The primary end points for the phase 1 portion were safety, tolerability, and ORR by RECIST 1.1 criteria for the phase 2 portion. Secondary end points included PFS, ORR, and DOR by RECIST 1.1 criteria, quality-of-life measures, and safety.
What has previously been reported?
In April 2023,
When the regimen was given in any line (n = 63), the ORR was 30%; 19 patients experienced a PR. Here, the DCR was 87% and the median PFS was about 7 months. In the group of patients who received the regimen in the first- and any-line but had hepatic-only disease (n = 20), the confirmed ORR was 35%. The DCR was 100% and the median PFS was approximately 11 months.
The median patient age was 64.5 years, with half of patients younger than 65 years and the other half 65 years and older. Most patients were White (93.2%), 52.3% were male, and more than half (61.4%) had an ECOG performance status of 0. Baseline lactate dehydrogenase level was normal for 65.9% of patients. The largest metastatic lesion was no larger than 3.0 cm for 54.5% of patients, 3.1 cm to 8.0 cm for 34.1% of patients, and 8.1 cm or larger for 9.1% of patients. Location of metastases were hepatic only for 52.3% of patients, extrahepatic only for 4.5% of patients, and both for 40.9% of patients. In terms of HLA-A2*02:01 status, 68.2% of patients were negative and 27.3% were positive; this was unknown for 4.5% of patients.
The median dose intensity for darovasertib was 92.6% and 88.0% for crizotinib. Moreover, the mean duration of exposure to darovasertib was 10.0 months. Treatment-related adverse effects (TRAEs) of any grade were experienced by 97.7% of patients who received the doublet; they were grade 3 or higher for 27.3% of patients. Treatment-related serious adverse effects were experienced by 9.1% of patients and were grade 3 or higher for 6.8% of cases. TRAEs resulted in discontinuation for 4.5% of patients.
The most common TRAEs experienced by more than 30% of patients who received the combination were diarrhea (all grade, 90.9%; grade ≥3, 2.3%), nausea (79.5%; 0%), peripheral edema (61.4%; 0%), vomiting (47.7%; 0%), dermatitis acneiform (43.2%; 0%), hypoalbuminemia (43.2%; 2.3%), and fatigue (38.6%; 0%).
“These first reported OS data and broader clinical efficacy observed with a manageable safety profile underscores the potential of the darovasertib and crizotinib combination in the first-line treatment landscape for patients with metastatic uveal melanoma,” Darrin Beaupre, MD, PhD, chief medical officer of IDEAYA Biosciences, stated in a news release.2
In April 2025, the
The phase 2/3 OptimUM-02 trial will evaluate the safety, tolerability, pharmacokinetics, and antitumor activity of darovasertib plus crizotinib in patients with HLA-A*02:01–negative metastatic uveal melanoma.6 The regimen will be compared with investigator’s choice of treatment, which could include pembrolizumab (Keytruda), ipilimumab (Yervoy) plus nivolumab (Opdivo), or dacarbazine.

Kaouther Ben Hania’s Gaza Drama, The Voice of Hind Rajab, has landed a Dec. 17 theatrical release in New York (Film Forum) and Los Angeles (Laemmle Theatres) with a national roll-out to follow via the distribution arm of the pic’s…
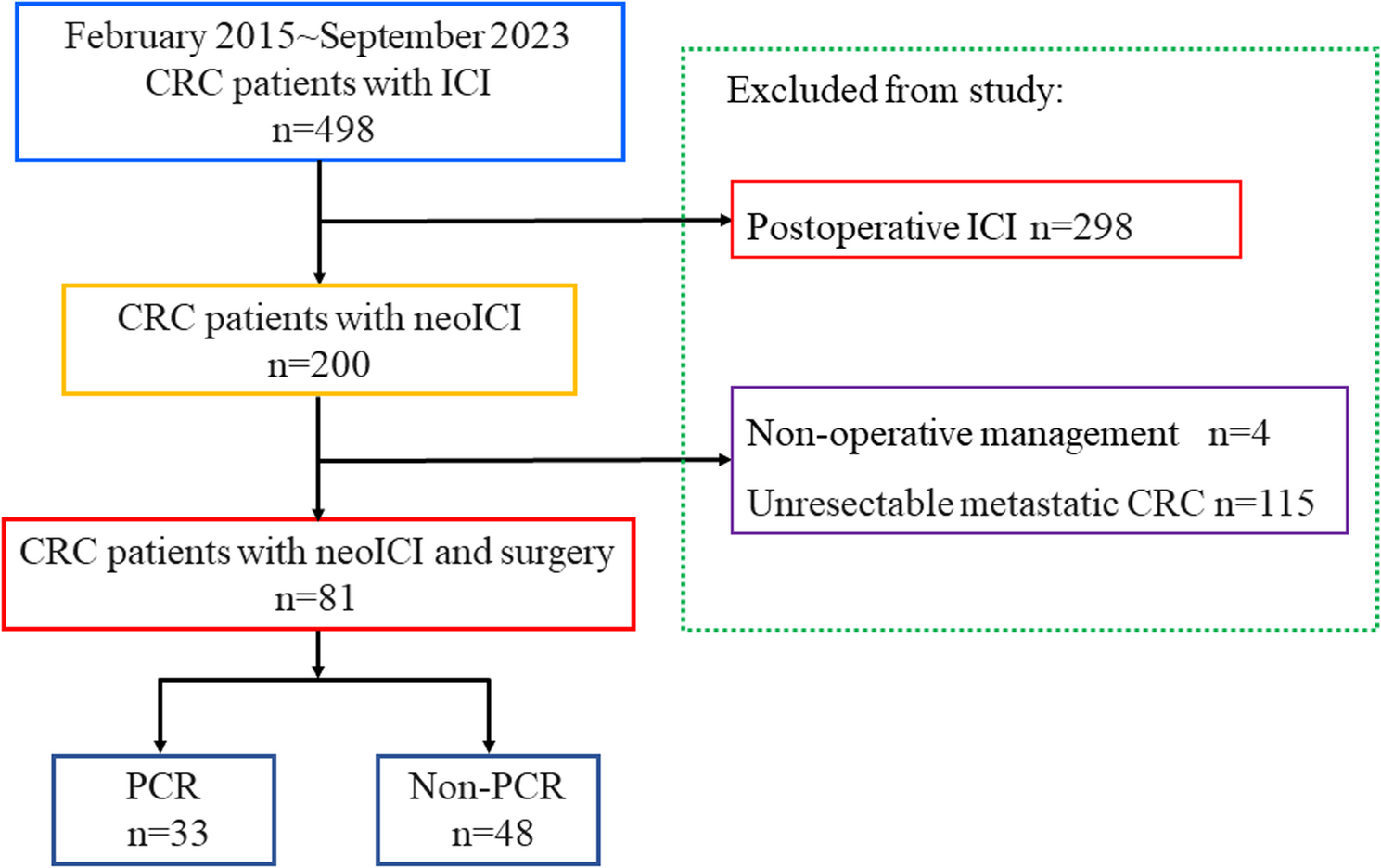
In this single-center retrospective study, all patients received immunotherapy before surgery. After surgery, more than 40% of the patients achieved pCR, with 66.7% and 29.8% of patients with dMMR and pMMR colorectal cancer achieving pCR,…

The Arctic Ocean is losing sea ice, and that is bad news for the climate. Yet a new paper reports a twist that adds nitrogen to these waters, which can feed algae that start the marine food chain.
Researchers measured a steady trickle of nitrogen…