The world’s most-visited museum will remain closed for the day for ‘exceptional reasons’.
Thieves wielding power tools have struck at the famed Louvre Museum in Paris, stealing eight items of priceless jewellery in a brazen heist that took…

The world’s most-visited museum will remain closed for the day for ‘exceptional reasons’.
Thieves wielding power tools have struck at the famed Louvre Museum in Paris, stealing eight items of priceless jewellery in a brazen heist that took…

The robots, each weighing 3.3 pounds or less, went head-to-head at the UK Beetle Championship held at St Michael’s Centre in Stoke Gifford, Bristol.
The event was organized by the Bristol Bot Builders, a group dedicated to sparking…

Tired of living in the land of auto-renew? Same—because even something as basic as watching a movie often starts with logging into Netflix, Max, or whatever subscription you added last month.
If you’re tired of the ever-growing amount of…
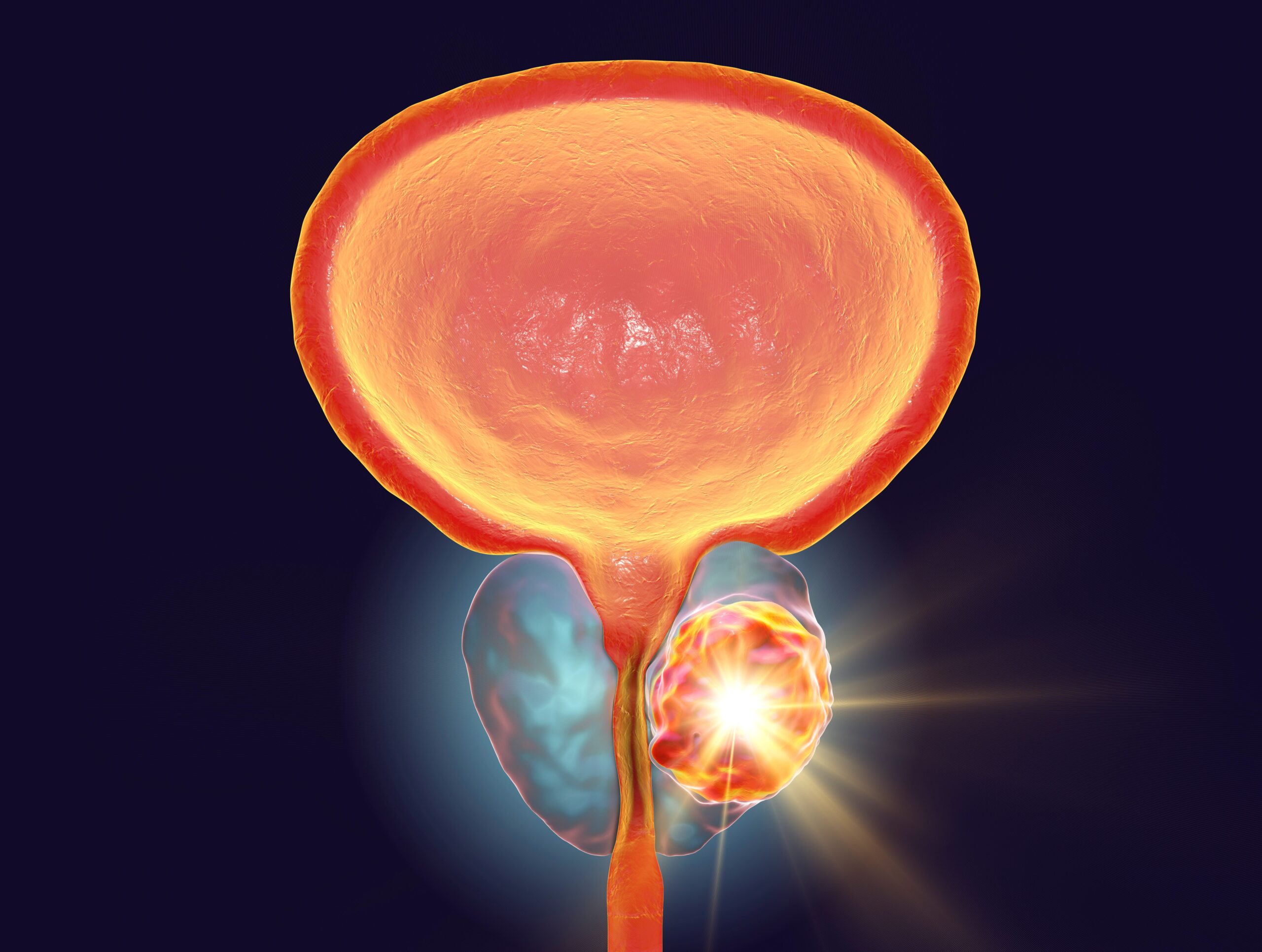
Enzalutamide (Xtandi) plus leuprolide acetate exhibited a 40.3% lower risk of death compared with leuprolide acetate alone in patients with high-risk, biochemically recurrent prostate cancer, according to findings from the final overall analysis…
Durvalumab (Imfinzi) in combination with chemotherapy produced outcomes consistent with prior studies in patients with advanced pleural mesothelioma, demonstrating favorable overall survival (OS), progression-free survival (PFS), and objective response rate (ORR) trends, according to findings from the phase 3 DREAM3R trial (NCT04334759) presented at the
Data were presented at the 2025 European Society for Medical Oncology (ESMO) Congress. Durvalumab and chemotherapy yielded a median OS of 21 months (95% CI, 18–27) vs 18 months for chemotherapy alone (95% CI, 14–30) for a HR of 0.92 (95% CI, 0.63–1.36; stratified log-rank P =.9). Median PFS were 8 months (95% CI, 8–10) with the combination vs 7 months (95% CI, 6–8) with chemotherapy alone (HR, 0.70; 95% CI, 0.5–0.98; P =.20). ORRs were 58% and 35%, respectively (P =.005).
The safety profiles of chemotherapy with or without durvalumab were consistent with the known profiles of the agents. Fatigue, nausea, and anemia were the most common adverse events (AEs) in both arms.
Durvalumab was assessed in the phase 2 DREAM (ACTRN12616001170415) and PrE0505 (NCT02899195). In these studies, durvalumab showed promising activity in combination with pemetrexed and either cisplatin or carboplatin chemotherapy.
The phase 3 DREAM3R study randomized patients 2:1 to receive durvalumab 1500 mg every 3 weeks plus chemotherapy every 3 weeks for 4 to 6 cycles, followed by durvalumab maintenance 1500 mg every 4 weeks until progressive disease or unacceptable toxicity, or chemotherapy every 3 weeks for 4 to 6 cycles. Accrual started in February 2021.
However, the results of the CheckMate 743 study (NCT02899299) published in 2021 showed that nivolumab (Opdivo) and ipilimumab (Yervoy) significantly improved OS vs chemotherapy.2 As the standard of care in pleural mesothelioma had changed, the study design changed to incorporate a nivolumab/ipilimumab arm, and randomization changed to 1:1 durvalumab/chemotherapy or physician’s choice of nivolumab/ipilimumab or chemotherapy.1 At this ESMO Congress, only data from the durvalumab and chemotherapy cohorts were presented.
A total of 114 patients received chemotherapy and durvalumab and 60 patients received chemotherapy alone. The study’s primary end point was OS. Secondary end points included PFS, ORR, and AEs.
During her presentation, Anna Nowak, MD, deputy vice chancellor at the University of Western Australia, noted that, while DREAM3R was a well-designed study, due to the early stopping of DREAM3R, it is unlikely that the study question will ever be answered.
“The key limitation is that slow accrual led to early stopping, and this slow accrual was no doubt, an artifact, partly of the global pandemic, but also then of a change of clinical practice as a result of the of the CheckMate 743 study and subsequent regulatory approval and standard clinical care use,” Nowak explained. “These design modifications add complexity to the interpretation of this clinical trial, and of course, chemotherapy alone would no longer be considered standard of care.”
Nowak did note that the high objective tumor response and control observed with chemotherapy and durvalumab raised the potential for future testing in the neoadjuvant setting. Additionally, correlative biomarkers that were collected from DREAM3R are also under ongoing analysis.
Further, Nowak emphasized the importance of timely activation of randomized phase 3 trials after positive phase 2 trials “is critically important to seize the window of opportunity for accrual and provide reliable answers.” Nowak added that, “the reasons for the delay in starting this trial were multifactorial.”
Disclosures: Nowak declared participation on AstraZeneca Data Safety Monitoring Board, board membership on Cancer Council Western Australia, position on Cancer Australia Advisory Council, and president-elect of the International Mesothelioma Interest Group.
1. Nowak A. Primary results of DREAM3R: DuRvalumab (MEDI4736) with chemotherapy as first line treatment in advanced pleural Mesothelioma – A phase 3 Randomised trial. Presented at: 2025 ESMO Congress; October 17–20, 2025; Berlin, Germany. Abstract LBA104.
2. Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021 Jan 30;397(10272):375-386. doi: 10.1016/S0140-6736(20)32714-8. Epub 2021 Jan 21.

Max Verstappen made a perfect start to lead Lap 1 at the United States Grand Prix, continuing as he did in Saturday’s Sprint.
Verstappen maintained the lead on the opening tour after starting from pole position for the second time this weekend,…

Archaeologists in western Bohemia announced a discovery of about 500 gold and silver Celtic coins, plus gold and bronze jewelry, spanning roughly the 6th to 1st centuries B.C..
Many of the coins are tiny, about 0.28 to 0.59 inches across, yet…


Sirexatamab (DKN-01) plus bevacizumab (Avastin) and standard-of-care (SOC) chemotherapy was safe and tolerable compared with bevacizumab and SOC in patients with metastatic colorectal carcinoma (mCRC). In particular, patients in the DKK1-high subgroup demonstrated a benefit in progression-free survival (PFS) and overall survival (OS), according to findings of the phase 2 DeFianCe trial (NCT05480306), presented at the 2025 European Society for Medical Oncology (ESMO) Congress.1
In the intent-to-treat population, patients who received the DKK1 inhibiting IgG4 antibody sirexatamab demonstrated an overall response rate (ORR) of 35.1% (95% CI, 25.5%-45.6%) vs 26.6% (95% CI, 18.0%-36.7%) for the control arm (P = .10). Median PFS was 9.2 months in the experimental arm vs 8.3 months in the control arm (P = .17).
“Neither of these 2 outcomes reached statistical significance,” lead author Zev A. Wainberg, MD, said during the presentation of data. “However, looking at the DKK1-high population, defined as the upper median, the improvement started to bear fruit,” Wainberg, professor of medicine at UCLA and codirector, UCLA GI program, at UCLA Health in California continued.
There were 50 patients with higher DKK1 in the sirexatamab arm and 38 in the control arm. The ORR in the sirexatamab arm was 38.0% (95% CI, 24.7%-52.8%) vs 23.7% (95% CI, 11.4%-40.2%) in the control arm (P = .0706). Median PFS in the experimental arm was 9.03 months vs 7.06 months (HR, 0.61; 95% CI, 0.37-1.0; P = .0255). Median OS was not reached in the experimental arm vs 14.39 months in the control arm (HR, 0.42; 95% CI, 0.19-0.91; P = .0118).
After an initial safety run-in period (n = 33), a total of 188 patients with mCRC were randomly assigned 1:1 to receive either sirexatamab plus either leucovorin, 5-fluorouracil (5-FU), and irinotecan (FOLFIRI) or leucovorin, 5-FU, and oxaliplatin (FOLFOX) and bevacizumab (n = 94) vs FOLFIRI or FOLFOX and bevacizumab (n = 94).
Patients were eligible if they had received 1 prior 5-FU–based therapy, had microsatellite stable (MSS) CRC, and did not have a BRAF V600 mutation. The stratification factors were tumor sidedness (left vs right) and prior antiangiogenesis therapy (yes vs no).
The primary end point was investigator-assessed PFS and the secondary end points were safety, ORR, and OS. Key exploratory end points were PFS, ORR, and the OS of the DKK1-high subgroup.
The study was well-balanced for gender and region. The majority of patients were male across the arms (experimental, 68%; control, 55%). In the experimental arm, the majority of patients were from South Korea (53%) compared with 48% in the control arm. Forty-four percent of patients were from the United States in the experimental arm and 43% in the control arm. The majority of patients had left-sided tumors (both 75%).
“This was a predominantly liver-metastatic patient population, with nearly 75% in both arms having liver metastatic disease,” Wainberg said. Further, 47% of patients in the experimental arm had RAS-mutated disease compared with 57% in the control arm. Looking at prior systemic therapy, 48% of patients in the experimental arm had received antiangiogenesis therapy compared with 51% in the control arm.
The overall rate of treatment-emergent adverse events (TEAEs) for sirexatamab was similar to the chemotherapy arm, suggesting that the addition of the agent does not adversely impact the safety profile of the combined agents.
In the experimental arm, 15% of patients discontinued therapy compared with 19% in the control arm. In the patients with DKK1-high status, 4% discontinued in the treatment arm vs not applicable in the control arm. Dose reductions affected 37% of patients in the experimental arm compared with 43% in the control arm. Dose interruptions were similar, with 73% in the experimental arm and 71% in the control arm.
“These data support continued development of sirexatamab in DKK1-high previously treated patients with mCRC,” Wainberg concluded.
DISCLOSURES: Dr Wainberg disclosed that he has provided consulting services to Alligator Therapeutics, Amgen, AstraZeneca, Arcus, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo Company, Eli Lilly and Company, EMD Serono, Roche AG, Genentech, Ipsen, Johnson & Johnson, Merus NV, Merck, Novartis, Novocure, Pfizer, Servier, and Verastem.