For decades, Mars has tempted scientists with its icy surface and quiet promise of life.
Now, a new study suggests that fragments of ancient microbes might still rest frozen in Martian ice, preserved far longer than anyone imagined, and surviving…

For decades, Mars has tempted scientists with its icy surface and quiet promise of life.
Now, a new study suggests that fragments of ancient microbes might still rest frozen in Martian ice, preserved far longer than anyone imagined, and surviving…
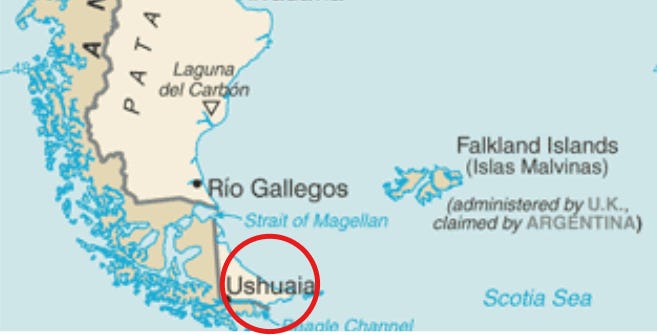
A whooping cough, or pertussis outbreak is reported in the capital city of Tiera del Fuego, Ushuaia, in Argentina.
To date, 58 confirmed cases have been reported, out of 135 suspected cases and more than 700 close contacts, in the capital of…
LOS ANGELES, Oct. 20 (Xinhua) — About nine in 10 U.S. adults have not heard of cardiovascular-kidney-metabolic (CKM) syndrome, a newly defined health condition that affects nearly 90 percent of U.S. adults, local media reported Monday, citing…

Photo-Illustration: by The Cut; Photo: Retailer
Bleu de Chanel is inescapable. Since the original…

Noah Hawley’s new FX series Alien: Earth doesn’t waste time pretending it’s just about xenomorphs. The monsters are there — acid blood, drooling menace, all that good stuff — but the real…
Pharmacists have many pivotal roles during respiratory virus season, including administering vaccines and counseling patients about viral respiratory infections, risk factors, and potential complications. Pharmacists can also utilize their…

Here’s what’s launching from Oct. 20 to Oct. 26: A new secure communications satellite for Spain and four other missions from the U.S. and China.

Immunotherapy (IO) and tyrosine kinase inhibitor (TKI) combination regimens have dramatically changed outcomes for patients with metastatic clear cell renal cell carcinoma (RCC). Now, clinical experience and research are providing better guidance on how to respond when patients’ disease recurs after the use of combination therapy. In a virtual Case-Based Roundtable event for oncologists in Oklahoma and Texas, moderator Nizar M. Tannir, MD, professor of genitourinary medical oncology at The University of Texas MD Anderson Cancer Center in Houston, presented data on second-line treatment and beyond and asked participants to share their experiences. They discussed how many of their patients are able to delay subsequent treatment for several years thanks to frontline combinations and also considered the barriers to use for the newer agents tivozanib (Fotivda) and belzutifan (Welireg).
DISCUSSION QUESTIONS
Nizar M. Tannir, MD: Vis-a-vis the combination arm; we already know that the combination should not be given after a prior therapy with nivolumab/ipilimumab or an IO/TKI. But what do you take out of this monotherapy arm with 9.2 months’ median progression-free survival [PFS] in patients who received 1.34 mg tivozanib as second line?1 Do you consider these results to be clinically meaningful, practice changing, or practice confirming?
Stephen Barman, MD: I think it depends on what you use in the first line. I use a lot of nivolumab plus ipilimumab [Yervoy] in the first line, unless…I need a very quick response in the first line, [or] somebody’s got significant hypoxia from lung disease; then I like to use a TKI. I still like cabozantinib [Cabometyx] in the second line and tivozanib more in the third line. Do you think that the FDA will update the indication in the second line?
Tannir: You bring up a very important point. Tivozanib is approved after at least 2 [prior therapies]. It’s not approved after only 1 line. The approval is based on the TIVO-3 trial [NCT02627963] vs sorafenib [Nexavar], and that trial required patients to have had 2 or 3 prior lines. Only 26% of those patients had [received] immune checkpoint inhibitors.2 The approval and the package insert [require that the patient] had to have received 2 lines, meaning the patient had to have an IO and a TKI.3 So…nivolumab/ipilimumab is considered dual immunotherapy in 1 line. Technically speaking, you’re going to have a hard time with insurance approving tivozanib after nivolumab/ipilimumab.
This is an important question. I think the data are impressive for tivozanib as a second line after nivolumab/ipilimumab with an 32% objective response rate and median PFS of 9.2 months.1 I like to prescribe this regimen because I like to give my patients an agent that’s well tolerated. When you look at hand-foot syndrome, mucositis, fatigue—except for hypertension—tivozanib doesn’t produce much of that compared with cabozantinib or lenvatinib [Lenvima] plus everolimus [Afinitor]. Even axitinib [Inlyta] causes more mucositis and hand-foot syndrome and liver toxicity, etc. Tivozanib, despite its long half-life, is very well tolerated compared with the other TKIs.
Rebecca Yarborough, MD: I have an older, frailer population as well, so I don’t use as much combination immunotherapy in my patients, unless they’re fitter or younger, and sometimes with more aggressive pathologies, such as sarcomatoid…. I use a lot of IO/TKI in the first line. A lot of times I go to cabozantinib in the second line. I’ve been in practice for 3 or 4 years, so I don’t have any patients beyond second line in my practice right now, so I haven’t had a chance to use tivozanib or belzutifan yet. I think it was very interesting to see the tivozanib data…. I would like to be able to use that more. I’m interested in its better tolerability compared with cabozantinib.
Parth Khade, MD: I thought it was significant in the sense that it favors supporting monotherapy as opposed to immune checkpoint inhibition rechallenge with the combination arm, resulting in slightly lower median PFS, but I think it also supports using tivozanib as monotherapy [with] the expected response rate in a multicenter trial looking at a diverse patient population. I think it gives good evidence that tivozanib is effective. The question just becomes what line of therapy to use it in, whether it’s third line, second line, etc.
Tannir: I can tell you, the earlier line you use any TKI is going to be a better result. If you wait for third or fourth line, your efficacy is going to drop, or radiographic PFS will be [shorter].
DISCUSSION QUESTIONS
Dai Chu Luu, MD: I think this is just practice confirming. When I think about how to choose between the 2, I’d pick tivozanib; it seems to be better tolerated. Of course, [belzutifan] was studied in a later line. With belzutifan it looks like anemia was a pretty big adverse event, whereas tivozanib seem to be well tolerated.1,4
Khusroo Qureshi, MD: All these things are very impressive. When I started [practicing] oncology and was in fellowship 25 years ago, we used to use IL-2, so when you compare it with interleukin therapy, all these things are very impressive. At Texas Oncology, we use a lot of pathways to guide us. My frontline therapy often is axitinib and pembrolizumab [Keytruda]. I’ve had patients tolerate axitinib quite well. Sometimes I’ve had issues with skin rash or diarrhea, and I just have them hold it if it’s really bad or just skip the weekends and it’s pretty well tolerated. Right now, I think I have 4 or 5 patients on first-line therapy for metastatic RCC, and they’re all doing really well. Some of them have been on therapy for over 1 year. For second-line therapy, cabozantinib would still be my preferred [option].
The data…including the singlet [tivozanib] vs tivozanib plus IO are very convincing, but it’s still not approved for second-line therapy, and we get a lot of pushback regarding whether something is on Compendia and whether it’s approved or not. Unless there’s a change, I probably would still hold tivozanib for the third line. As far as fourth line and belzutifan…I’ve not had a chance to use that. In the past, when I’ve had patients reach that point, I’ve sent patients to my colleague who runs our phase 1 research programs and I send patients to Mary Crowley Cancer Research [in Dallas, Texas] to see what else is available. But so far, I’ve had an excellent response on my first-line therapies, so I have not even had a chance to use a second line in the last 18 to 24 months.
Ofobuike Okani, MD: I have the same experience at Texas Oncology. I treated a 68-year-old patient close to 3 years ago, and he had clear cell metastatic RCC. He received 24 months of pembrolizumab/axitinib and I stopped at 24 months. He still has no evidence of disease on all his scans. I treated another 89-year-old patient who couldn’t tolerate pembrolizumab/axitinib. He had liver toxicity which was pretty bad, so I kept him on only axitinib, and he’s doing well at 89 years old. Another patient with metastatic clear cell RCC is on maintenance pembrolizumab for close to 9 months. I have another patient who is in his 40s, who is getting axitinib/pembrolizumab, and he’s doing very well. The only patient I have treated with second-line cabozantinib was a patient I treated with The University of Texas MD Anderson Cancer Center, but other than that, I’ve never gone beyond the second line, so I have not used tivozanib. I have not had any experience with any of these agents treating beyond the second line.
DISCLOSURES: Tannir previously reported research funding from Bristol-Myers Squibb Company (BMS), Calithera Biosciences, Nektar Therapeutics, Exelixis, Pfizer, Novartis, Arrowhead Pharmaceuticals, Mirati Therapeutics, Takeda, Epizyme, and Eisai Medical Research; consulting, advisory, travel accommodations, and expenses from BMS, Calithera Biosciences, Nektar Therapeutics, Exelixis, Pfizer, Novartis, Eisai Medical Research, Ipsen, Lilly Oncology, Neoleukin Therapeutics, Surface Oncology, ONO Pharmaceutical, and Oncorena; and honoraria from BMS, Exelixis, Nektar Therapeutics, Calithera Biosciences, Eisai Medical Research, ONO Pharmaceutical, Eli Lilly, Oncorena, Ipsen, and Surface Oncology.
References:
1. Choueiri TK, Albiges L, Barthélémy P, et al. Tivozanib plus nivolumab versus tivozanib monotherapy in patients with renal cell carcinoma following an immune checkpoint inhibitor: results of the phase 3 TiNivo-2 study. Lancet. 2024;404(10460):1309-1320. doi:10.1016/S0140-6736(24)01758-6
2. Rini BI, Pal SK, Escudier BJ, et al. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. 2020;21(1):95-104. doi:10.1016/S1470-2045(19)30735-1
3. Fotivda. Prescribing information. Aveo Pharmaceuticals; 2021. Accessed October 20, 2025.
4. Choueiri TK, Powles T, Peltola K, et al. Belzutifan versus everolimus for advanced renal-cell carcinoma. N Engl J Med. 2024;391(8):710-721. doi:10.1056/NEJMoa2313906

– Additional Phase 3 PURPOSE data reinforce the safety profile of twice-yearly Yeztugo® as an effective HIV prevention option across diverse populations –
– New data show higher treatment satisfaction after switching from IM CAB+RPV to Biktarvy® –
– New research reaffirms Veklury® in high-risk COVID-19 populations and obeldesivir in emerging pathogens –
Source : Gilead Sciences, Inc.

Periodic Labs, a new startup by one of OpenAI’s most respected researchers, Liam Fedus, and his former Google Brain colleague, Ekin Dogus Cubuk, came out of stealth last month with an enormous $300 million seed round. It was led by Felicis…