Sunderam S, Kissin DM, Zhang Y, Jewett A, Boulet SL, Warner L, et al. Assisted reproductive technology surveillance – United States, 2018. MMWR Surveill Summ. 2022;71(4):1–19.
Google Scholar
Bai F, Wang DY, Fan YJ, Qiu J, Wang L, Dai Y, et al. Assisted reproductive technology service availability, efficacy and safety in mainland china: 2016. Hum Reprod. 2020;35(2):446–52.
Google Scholar
Wyns C, De Geyter C, Calhaz-Jorge C, Kupka MS, Motrenko T, Smeenk J, et al. ART in Europe, 2018: results generated from European registries by ESHRE. Hum Reprod Open. 2022;2022(3):hoac022.
Google Scholar
Banker M, Dyer S, Chambers GM, Ishihara O, Kupka M, de Mouzon J, et al. International committee for monitoring assisted reproductive technologies (ICMART): world report on assisted reproductive technologies, 2013. Fertil Steril. 2021;116(3):741–56.
Google Scholar
Chambers GM, Dyer S, Zegers-Hochschild F, de Mouzon J, Ishihara O, Banker M, et al. International committee for monitoring assisted reproductive technologies world report: assisted reproductive technology, 2014. Hum Reprod. 2021;36(11):2921–34.
Google Scholar
Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23(2):139–55.
Google Scholar
Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil Steril. 2014;102(1):19–26.
Google Scholar
Wei D, Liu JY, Sun Y, Shi Y, Zhang B, Liu JQ, et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet. 2019;393(10178):1310–8.
Google Scholar
Wiegel RE, Jan Danser AH, Steegers-Theunissen RPM, Laven JSE, Willemsen SP, Baker VL, et al. Determinants of maternal Renin-Angiotensin-Aldosterone-System activation in early pregnancy: insights from 2 cohorts. J Clin Endocrinol Metab. 2020;105(11):3505–17.
Google Scholar
Koerts JJ, Voskamp LW, Rousian M, Steegers-Theunissen RPM, Wiegel RE. Impact of corpus luteum number on maternal pregnancy and birth outcomes: the Rotterdam periconception cohort. Fertil Steril. 2025;123(6):1039–50.
Google Scholar
Zaat TR, Kostova EB, Korsen P, Showell MG, Mol F, van Wely M. Obstetric and neonatal outcomes after natural versus artificial cycle frozen embryo transfer and the role of luteal phase support: a systematic review and meta-analysis. Hum Reprod Update. 2023;29(5):634–54.
Google Scholar
Roelens C, Blockeel C. Impact of different endometrial preparation protocols before frozen embryo transfer on pregnancy outcomes: a review. Fertil Steril. 2022;118(5):820–7.
Google Scholar
Ho VNA, Pham TD, Nguyen NT, Wang R, Norman RJ, Mol BW, et al. Livebirth rate after one frozen embryo transfer in ovulatory women starting with natural, modified natural, or artificial endometrial preparation in Viet nam: an open-label randomised controlled trial. Lancet. 2024;404(10449):266–75.
Google Scholar
Ghobara T, Gelbaya TA, Ayeleke RO. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst Rev. 2017;7(7):Cd003414.
Google Scholar
Busnelli A, Schirripa I, Fedele F, Bulfoni A, Levi-Setti PE. Obstetric and perinatal outcomes following programmed compared to natural frozen-thawed embryo transfer cycles: a systematic review and meta-analysis. Hum Reprod. 2022;37(7):1619–41.
Google Scholar
Yuan Y, Chang Q, Wen Y, Gao J, Huang S, Xu Y, et al. Letrozole during frozen embryo transfer in women with polycystic ovarian syndrome: a randomized controlled trial. Obstet Gynecol. 2023;142(5):1087–95.
Google Scholar
Salemi S, Yahyaei A, Vesali S, Ghaffari F. Endometrial preparation for vitrified-warmed embryo transfer with or without GnRH-agonist pre-treatment in patients with polycystic ovary syndrome: a randomized controlled trial. Reprod Biomed Online. 2021;43(3):446–52.
Google Scholar
Li J, Lin Z, Mo S, Wang S, Li Y, Shi Q. Pretreatment with or without GnRH-agonist before frozen-thawed embryo transfer in patients with PCOS: a systematic review and meta-analysis. J Ovarian Res. 2024;17(1):130.
Google Scholar
Niu Z, Chen Q, Sun Y, Feng Y. Long-term pituitary downregulation before frozen embryo transfer could improve pregnancy outcomes in women with adenomyosis. Gynecol Endocrinol. 2013;29(12):1026–30.
Google Scholar
Guo Y, Fang Z, Yu L, Sun X, Li F, Jin L. Which endometrial preparation protocol provides better pregnancy and perinatal outcomes for endometriosis patients in frozen-thawed embryo transfer cycles? A retrospective study on 1413 patients. J Ovarian Res. 2023;16(1):7.
Google Scholar
Magnusson Å, Hanevik HI, Laivuori H, Loft A, Piltonen T, Pinborg A, et al. Endometrial preparation protocols prior to frozen embryo transfer – convenience or safety? Reprod Biomed Online. 2024;48(1):103587.
Google Scholar
Polyzos NP. Endometrial preparation protocols for frozen embryo transfer: risk assessment and individualized management. Hum Reprod. 2025;deaf149.
Hamze H, Alameh W, Hemmings R, Jamal W, Banan A, Sylvestre C. The science of frozen embryo transfer, is modified natural cycle better? Gynecol Endocrinol. 2025;41(1):2533481.
Google Scholar
Liu X, Li W, Wen W, Wang T, Wang T, Sun T, et al. Natural cycle versus hormone replacement therapy as endometrial preparation in ovulatory women undergoing frozen-thawed embryo transfer: the compete open-label randomized controlled trial. PLoS Med. 2025;22(6):e1004630.
Google Scholar
Huang J, Liao Y, Xia L, Wu H, Liu Z, Lin J, et al. Impact of endometrial preparation protocols on pregnancy outcomes in patients with unexplained recurrent implantation failure undergoing frozen embryo transfer. Ultrasound Obstet Gynecol. 2025;65(5):633–40.
Google Scholar
Guo Z, Chu R, Zhang L, Yu Q, Yan L, Ma J. Fresh versus frozen embryo transfer in women with thin endometrium: a retrospective cohort study. Ann Transl Med. 2020;8(21):1435.
Google Scholar
Stentz N, Devine K. Through thick and thin: time to stop worrying about endometrial thickness? Fertil Steril. 2021;116(1):71–2.
Google Scholar
Liu KE, Hartman M, Hartman A, Luo ZC, Mahutte N. The impact of a thin endometrial lining on fresh and frozen-thaw IVF outcomes: an analysis of over 40 000 embryo transfers. Hum Reprod. 2018;33(10):1883–8.
Google Scholar
Kasius A, Smit JG, Torrance HL, Eijkemans MJ, Mol BW, Opmeer BC, Broekmans FJ. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(4):530–41.
Google Scholar
Mahutte N, Hartman M, Meng L, Lanes A, Luo ZC, Liu KE. Optimal endometrial thickness in fresh and frozen-thaw in vitro fertilization cycles: an analysis of live birth rates from 96,000 autologous embryo transfers. Fertil Steril. 2022;117(4):792–800.
Google Scholar
Gallos ID, Khairy M, Chu J, Rajkhowa M, Tobias A, Campbell A, et al. Optimal endometrial thickness to maximize live births and minimize pregnancy losses: analysis of 25,767 fresh embryo transfers. Reprod Biomed Online. 2018;37(5):542–8.
Google Scholar
Fang Z, Huang J, Mao J, Yu L, Wang X. Effect of endometrial thickness on obstetric and neonatal outcomes in assisted reproduction: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2023;21(1):55.
Google Scholar
Ganer Herman H, Volodarsky-Perel A, Ton Nu TN, Machado-Gedeon A, Cui Y, Shaul J, Dahan MH. Pregnancy complications and placental histology following embryo transfer with a thinner endometrium. Hum Reprod. 2022;37(8):1739–45.
Google Scholar
Guo Z, Xu X, Zhang L, Zhang L, Yan L, Ma J. Endometrial thickness is associated with incidence of small-for-gestational-age infants in fresh in vitro fertilization-intracytoplasmic sperm injection and embryo transfer cycles. Fertil Steril. 2020;113(4):745–52.
Google Scholar
Oron G, Hiersch L, Rona S, Prag-Rosenberg R, Sapir O, Tuttnauer-Hamburger M, et al. Endometrial thickness of less than 7.5 mm is associated with obstetric complications in fresh IVF cycles: a retrospective cohort study. Reprod Biomed Online. 2018;37(3):341–8.
Google Scholar
Liu KE, Hartman M, Hartman A. Management of thin endometrium in assisted reproduction: a clinical practice guideline from the Canadian fertility and andrology society. Reprod Biomed Online. 2019;39(1):49–62.
Google Scholar
Wang P, Yang H, Chen Z, Chen Y, Jin C, Yu R, et al. Agonist long protocol improves outcomes of vitrified-warmed embryo transfer in repeatedly thin endometrium. Reprod Biomed Online. 2023;46(3):527–35.
Google Scholar
Ali J, Magray S, Ahmed E, Talo S. A case report of successful live pregnancy following embryo transfer in a thin endometrium. Cureus. 2024;16(8):e66363.
Google Scholar
Nagaraja N, Poddar SD, Rai S, Verma V, Abhisheka K, Khurana A. Improved pregnancy outcomes and endometrial receptivity by thawed frozen embryo transfer in mildly stimulated cycles with letrozole combined with estrogen in women with unresponsive thin endometrium compared to standard endometrial preparation with estrogen alone: a retrospective study. J Obstet Gynaecol India. 2023;73(4):351–7.
Google Scholar
Xia L, Tian L, Zhang S, Huang J, Wu Q. Hormonal replacement treatment for Frozen-Thawed embryo transfer with or without GnRH agonist pretreatment: A retrospective cohort study stratified by times of embryo implantation failures. Front Endocrinol (Lausanne). 2022;13:803471.
Hu X, Yan E, Peng W, Zhou Y, Jin L, Qian K. Higher pre-pregnancy body mass index was associated with adverse pregnancy and perinatal outcomes in women with polycystic ovary syndrome after a freeze-all strategy: a historical cohort study. Acta Obstet Gynecol Scand. 2024;103(5):884–96.
Google Scholar
Zhou R, Zhang X, Huang L, Wang S, Li L, Dong M, et al. The impact of different cycle regimens on birthweight of singletons in frozen-thawed embryo transfer cycles of ovulatory women. Fertil Steril. 2022;117(3):573–82.
Google Scholar
Roelens C, Racca A, Mackens S, Van Landuyt L, Buelinckx L, Gucciardo L, et al. Artificially prepared vitrified-warmed embryo transfer cycles are associated with an increased risk of pre-eclampsia. Reprod Biomed Online. 2022;44(5):915–22.
Google Scholar
Rodríguez-Eguren A, Bueno-Fernandez C, Gómez-Álvarez M, Francés-Herrero E, Pellicer A, Bellver J, et al. Evolution of biotechnological advances and regenerative therapies for endometrial disorders: a systematic review. Hum Reprod Update. 2024;30(5):584–613.
Google Scholar
Shang Y, Wu M, He R, Ye Y, Sun X. Administration of growth hormone improves endometrial function in women undergoing in vitro fertilization: a systematic review and meta-analysis. Hum Reprod Update. 2022;28(6):838–57.
Google Scholar
Yuan G, Yu C, Du X, Li D, Dou H, Lu P, et al. Injectable GelMA hydrogel microspheres with sustained release of Platelet-Rich plasma for the treatment of thin endometrium. Small. 2024;20(47):e2403890.
Google Scholar
Hu KL, Zhang D, Li R. Endometrium preparation and perinatal outcomes in women undergoing single-blastocyst transfer in frozen cycles. Fertil Steril. 2021;115(6):1487–94.
Google Scholar
Gu F, Wu Y, Tan M, Hu R, Chen Y, Li X, et al. Programmed frozen embryo transfer cycle increased risk of hypertensive disorders of pregnancy: a multicenter cohort study in ovulatory women. Am J Obstet Gynecol MFM. 2023;5(1):100752.
Google Scholar
Ginström Ernstad E, Wennerholm UB, Khatibi A, Petzold M, Bergh C. Neonatal and maternal outcome after frozen embryo transfer: increased risks in programmed cycles. Am J Obstet Gynecol. 2019;221(2):126.e1-e18.
Pereira MM, Mainigi M, Strauss JF. Secretory products of the corpus luteum and preeclampsia. Hum Reprod Update. 2021;27(4):651–72.
Google Scholar
von Versen-Höynck F, Narasimhan P, Selamet Tierney ES, Martinez N, Conrad KP, Baker VL, Winn VD. Absent or excessive corpus luteum number is associated with altered maternal vascular health in early pregnancy. Hypertension. 2019;73(3):680–90.
von Versen-Höynck F, Schaub AM, Chi YY, Chiu KH, Liu J, Lingis M, et al. Increased preeclampsia risk and reduced aortic compliance with in vitro fertilization cycles in the absence of a corpus luteum. Hypertension. 2019;73(3):640–9.
Matorras R, Pijoan JI, Laínz L, Díaz-Nuñez M, Sainz H, Pérez-Fernandez S, Moreira D. Polycystic ovarian syndrome and miscarriage in IVF: systematic revision of the literature and meta-analysis. Arch Gynecol Obstet. 2023;308(2):363–77.
Google Scholar
Vinsonneau L, Labrosse J, Porcu-Buisson G, Chevalier N, Galey J, Ahdad N, et al. Impact of endometrial preparation on early pregnancy loss and live birth rate after frozen embryo transfer: a large multicenter cohort study (14 421 frozen cycles). Hum Reprod Open. 2022;2022(2):hoac007.
Google Scholar
Hatoum I, Bellon L, Swierkowski N, Ouazana M, Bouba S, Fathallah K, et al. Disparities in reproductive outcomes according to the endometrial preparation protocol in frozen embryo transfer: the risk of early pregnancy loss in frozen embryo transfer cycles. J Assist Reprod Genet. 2018;35(3):425–9.
Google Scholar
Tomás C, Alsbjerg B, Martikainen H, Humaidan P. Pregnancy loss after frozen-embryo transfer–a comparison of three protocols. Fertil Steril. 2012;98(5):1165–9.
Google Scholar
Xu B, Geerts D, Hu S, Yue J, Li Z, Zhu G, Jin L. The depot GnRH agonist protocol improves the live birth rate per fresh embryo transfer cycle, but not the cumulative live birth rate in normal responders: a randomized controlled trial and molecular mechanism study. Hum Reprod. 2020;35(6):1306–18.
Google Scholar
Guo S, Li Z, Yan L, Sun Y, Feng Y. GnRH agonist improves pregnancy outcome in mice with induced adenomyosis by restoring endometrial receptivity. Drug Des Devel Ther. 2018;12:1621–31.
Ruan HC, Zhu XM, Luo Q, Liu AX, Qian YL, Zhou CY, et al. Ovarian stimulation with GnRH agonist, but not GnRH antagonist, partially restores the expression of endometrial integrin beta3 and leukaemia-inhibitory factor and improves uterine receptivity in mice. Hum Reprod. 2006;21(10):2521–9.
Google Scholar
Song J, Duan C, Cai W, Wu W, Lv H, Xu J. Comparison of GnRH-a prolonged protocol and short GnRH-a long protocol in patients with thin endometrium for assisted reproduction: A retrospective cohort study. Drug Des Devel Ther. 2020;14:3673–82.
Liu Y, Ma L, Zhu M, Yin H, Yan H, Shi M. Strobe-GnRHa pretreatment in frozen-embryo transfer cycles improves clinical outcomes for patients with persistent thin endometrium: a case-control study. Medicine. 2022;101(31):e29928.
Google Scholar
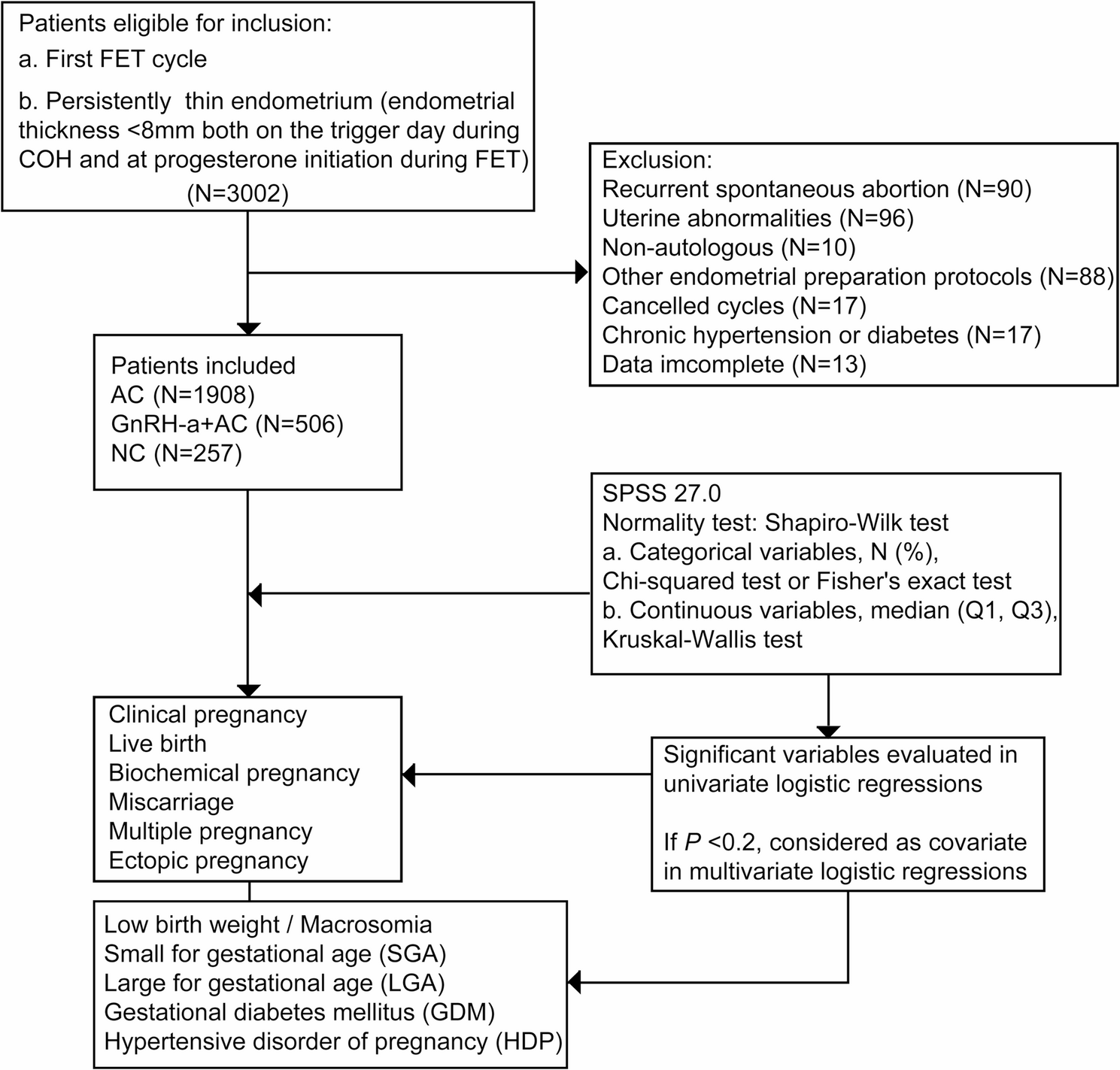






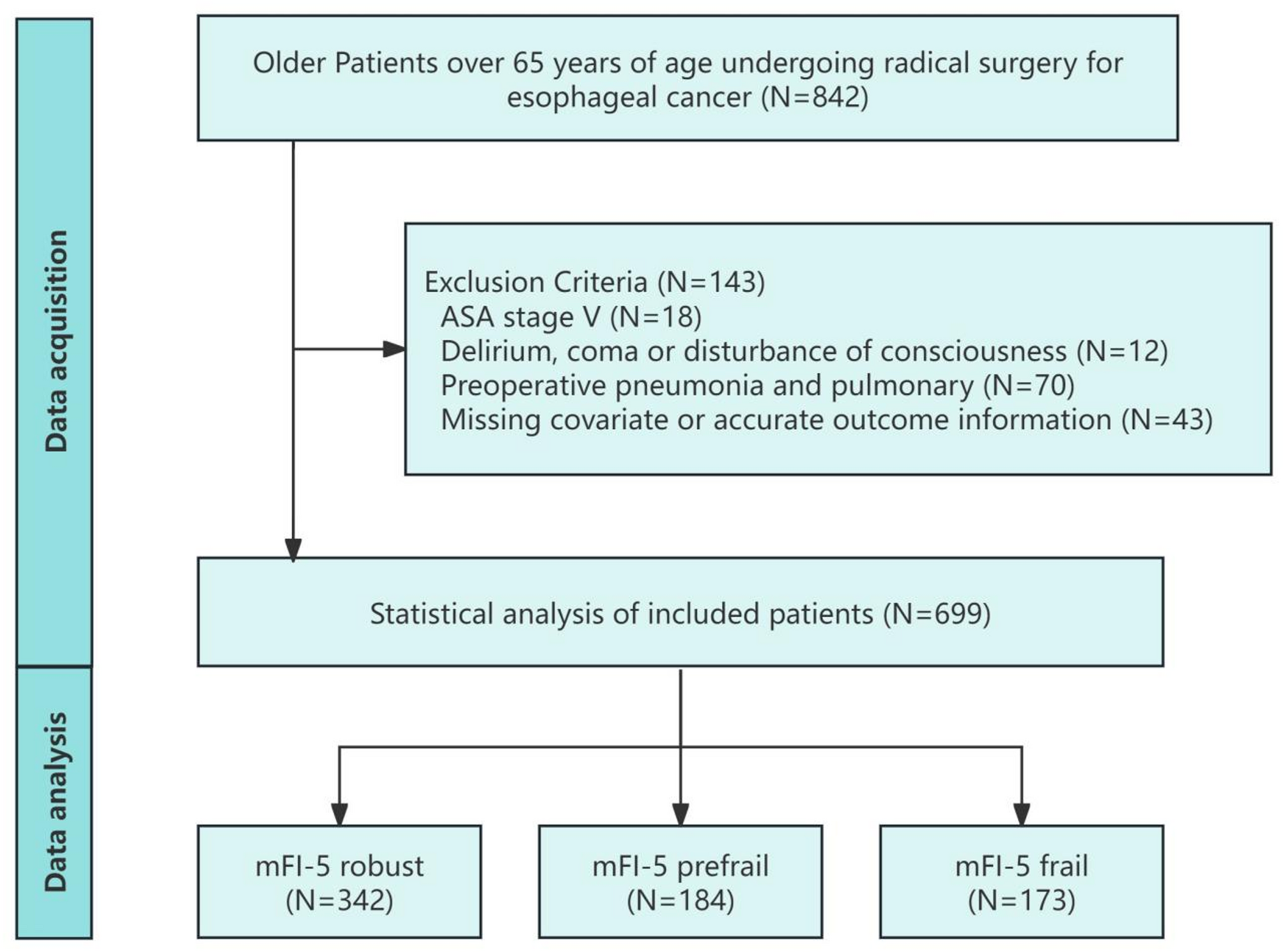

00263-8/asset/c1404d50-9e80-463d-9688-a16b7f125c81/main.assets/gr3.jpg)