Newly released images of the interstellar comet 3I/ATLAS appear to show the alien object spitting out an enormous jet of gas and dust toward the sun — just as comets are expected to do.
Discovered in late June and confirmed by NASA in early…
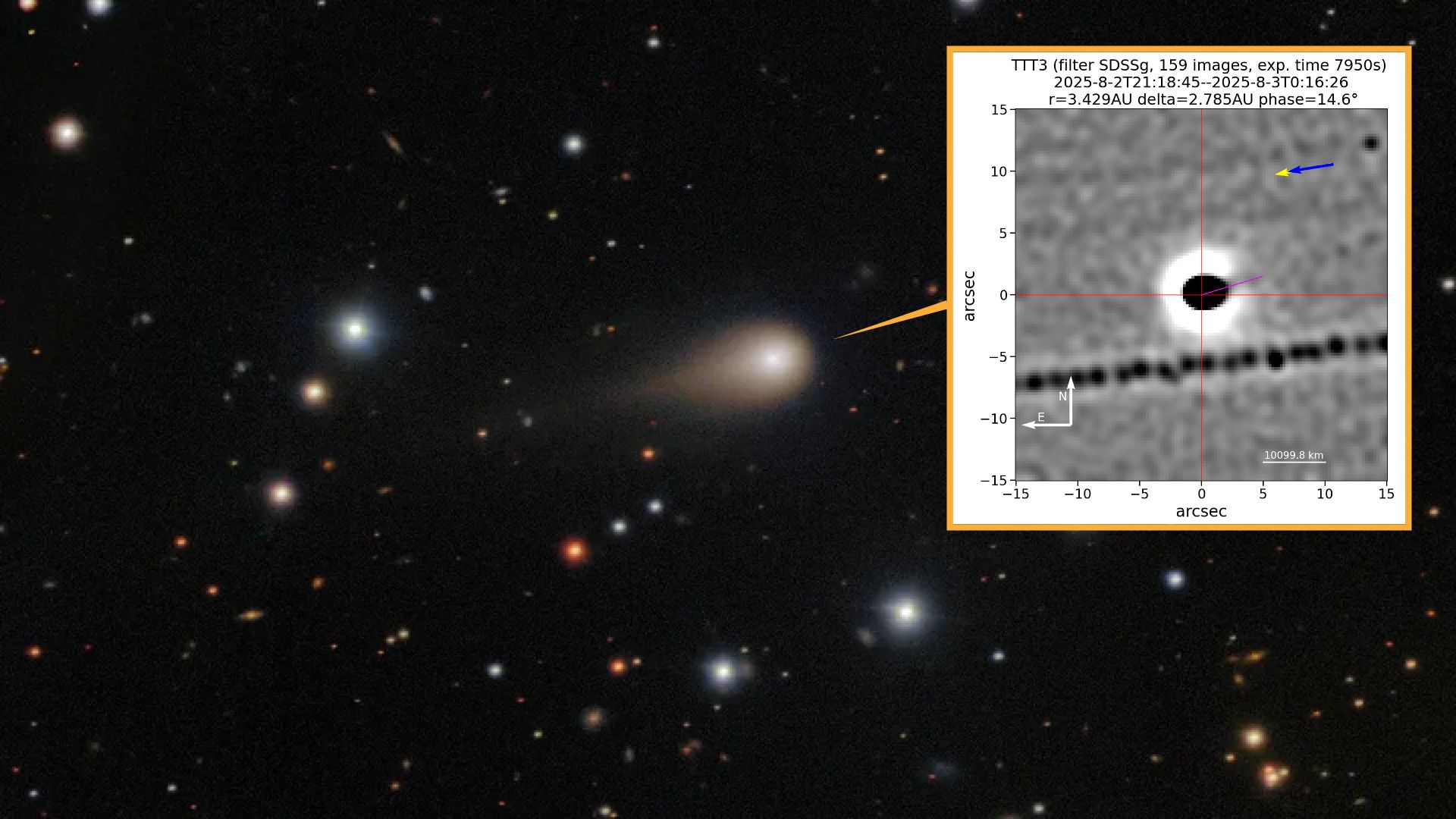
Newly released images of the interstellar comet 3I/ATLAS appear to show the alien object spitting out an enormous jet of gas and dust toward the sun — just as comets are expected to do.
Discovered in late June and confirmed by NASA in early…

Researchers have identified the largest known impact crater on Earth formed…
(Reuters) -AI data centre startup Crusoe is raising $1.38 billion at a valuation of about $10 billion from an anticipated Series E funding round, the Denver-based company said on Thursday
The oversubscribed round is being co-led by Valor Equity Partners and Mubadala Capital and includes Nvidia, Fidelity Management and Founders Fund as some of its major investors.
The company, which was involved in building OpenAI’s first big data centre in the U.S., has raised about $3.9 billion since its inception in 2018.
It recently announced that the first phase of its 1.2 gigawatt data center in Abilene, Texas campus, was live just one year after construction began.
(Reporting by Anuj T in Bengaluru; Editing by Anil D’Silva)

Michael van Gerwen survived a scare as he came back from 4-2 down to beat fellow Dutchman Wessel Nijman 6-5 in the first round of the European Championship.
Nijman failed to take seven match darts in the 11th leg before Van Gerwen eventually…

Formula 1 heads to Mexico City for Round 20 of the championship, but what do the odds say about who might come out on top? Read on to find out…
Odds are provided by F1’s Official Betting Data Supplier ALT Sports Data, are subject to change…

Netflix’s Black Rabbit took over the No. 1 spot on Nielsen’s streaming charts in its second week of release.
The drama starring Jude Law and Jason Bateman delivered 1.26 billion minutes of viewing for the week of…

American Ballet Theater doesn’t typically align its seasonal galas with a company member’s farewell, but in the case of Misty Copeland, whose trailblazing career has irrevocably changed the 85-year-old institution, an exception was made. A…

A trio of biomedical scientists at the University of California and University of Massachusetts have written a research-backed defense of DEI programs that was published today in the journal Nature Cell Biology. They assert that such…

The session will focus on strengthening PML-N,PPP relations at the provincial level
A meeting of the PPP and PML-N leadership. PHOTO: EXPRESS

The FDA has approved belantamab mafodotin-blmf (Blenrep) in combination with bortezomib (Velcade) and dexamethasone (BVd) for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least 2 prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent (IMID).1
The approval is supported by findings from the phase 3 DREAMM-7 (NCT04246047) trial. In the study, among patients who had received at least 2 prior lines of therapy, including a PI and an IMID, BVd elicited a 51% reduction in the risk of death (HR, 0.49; 95% CI, 0.32-0.76) compared with DVd. Treatment with the combination also led to a median progression-free survival (PFS) of 31.3 months (95% CI, 23.5-NR) vs 10.4 months (95% CI, 7.0-13.4) with DVd (HR, 0.31; 95% CI, 0.21-0.47). The safety and tolerability profiles of DVd and BVd were consistent with the known safety profiles of the individual agents, according to the news release.
“With the approval of [belantamab mafodotin], we now have a community-accessible BCMA-targeting agent with the potential to improve outcomes for patients following 2 or more prior lines of treatment, where options are limited,” Sagar Lonial, MD, chief medical officer and chair of the Emory Department of Hematology and Medical Oncology at the Winship Cancer Institute of Emory University in Atlanta, Georgia, stated in a news release “This approval marks an important advance in the US relapsed/refractory treatment landscape.”
This approval follows a divided
“Today’s FDA approval of [belantamab mafodotin] is another significant milestone, providing potential for superior efficacy, including overall survival, to US patients,” Tony Wood, chief scientific officer of GSK, added in the news release.1 “There is an urgent need for new and novel therapies, as nearly all patients with multiple myeloma experience relapse, and re-treating with the same mechanism of action often leads to suboptimal outcomes. As the only anti-BCMA agent that can be administered across health care settings, including in community centers where 70% of patients receive care, [belantamab mafodotin] fulfills a major patient need. We believe [belantamab mafodotin] can redefine treatment for patients with multiple myeloma in all parts of the world, and we are accelerating its development in earlier lines of therapy to support its use across all stages of this difficult-to-treat cancer.”
In the open-label DREAMM-7 trial, patients with relapsed/refractory multiple myeloma received either BVd or daratumumab (Darzalex) plus bortezomib and dexamethasone (DVd).3 Eligible patients had received at least 1 prior therapy but had not previously received a BCMA-targeted agent and were not refractory to anti-CD38 therapy.
Grade 3 or higher adverse effects (AEs) were more frequent in the BVd arm (95%) than in the DVd arm (78%). Ocular toxicity was more common in patients receiving belantamab mafodotin, occurring at a rate of 79% vs 29% in the DVd arm, but was largely reversible with dose modifications.
“The reality for most patients with multiple myeloma is a relentless cycle of remission and relapse, as their disease becomes refractory to treatments,” Michael Andreini, president and chief executive officer of the Multiple Myeloma Research Foundation and the Multiple Myeloma Research Consortium, added in the news release. “Patients urgently need more effective treatment options that can offer more quality time with their loved ones. We see the potential for [belantamab mafodotin] in combination to help patients achieve this.”