Max Verstappen has targeted improvements for Sunday’s United States Grand Prix, to ward off the unknown of McLaren’s race pace, despite controlling Saturday’s Sprint as he claimed another victory.
Verstappen closed to within 55 points of the…

Max Verstappen has targeted improvements for Sunday’s United States Grand Prix, to ward off the unknown of McLaren’s race pace, despite controlling Saturday’s Sprint as he claimed another victory.
Verstappen closed to within 55 points of the…

Perioperative enfortumab vedotin-ejfv (Padcev) plus pembrolizumab (Keytruda) with radical cystectomy and standard pelvic lymph node dissection (RC + PLND) significantly improved event-free survival (EFS), overall survival (OS), and pathologic complete response (pCR) rate vs RC + PLND followed by observation alone in patients with muscle-invasive bladder cancer (MIBC) who were not eligible for or refused cisplatin-based chemotherapy, according to data from the phase 3 KEYNOTE-905 study (NCT03924895).1
The data, which were shared during the
Top Takeaways from KEYNOTE-905:
Moreover, the median OS with enfortumab vedotin plus pembrolizumab was also NR (95% CI, NR-NR) vs 41.7 months (95% CI, 31.8-NR) with the control (HR, 0.50; 95% CI, 0.33-0.74; 1-sided P = .0002). The 12- and 24-month OS rates in the enfortumab arm were 86.3% and 79.7%, respectively; in the control arm, these rates were 75.7% and 63.1%. The pCR rate with enfortumab plus pembrolizumab was 57.1% (95% CI, 49.3%-64.6%) vs 8.6% (95% CI, 4.9%-13.8%) with the control, translating to an estimated difference of 48.3% (95% CI, 39.5%-56.5%) between arms (1-sided P < .000001).
“KEYNOTE-905 is the first phase 3 study to show improved efficacy outcomes with perioperative therapy relative to surgery for patients with MIBC who are ineligible for cisplatin-based chemotherapy,” Christof Vulsteke, MD, PhD, of the Integrated Cancer Center Ghent, AZ Maria Middelares, in Belgium, and the Center for Oncological Research at Antwerp University in Belgium, said in a presentation. “Perioperative enfortumab vedotin plus pembrolizumab added to RC + PLND may represent a new standard of care in this population with high unmet clinical need.”
The trial enrolled adult patients with MIBC who had an ECOG performance status ranging from 0 to 2, clinical stage T2 to T4aN0M0 or T1 to T4aN1M0 disease by central assessment, and at least 50% urothelial histology.1 Patients were either ineligible to receive cisplatin per Galsky criteria, or they had refused it.
Patients (n = 344) were randomly assigned 1:1 to receive pembrolizumab at 200 mg every 3 weeks (Q3W) for 3 cycles with enfortumab vedotin at 1.25 mg/kg on days 1 and 8 Q3W, then RC + PLND followed by adjuvant pembrolizumab at 200 mg Q3W for 14 cycles plus enfortumab vedotin at 1.25 mg/kg on days 1 and 8 Q3W (n = 170) or RC + PLND followed by observation (n = 174). They were stratified based on cisplatin ineligibility (ineligible vs eligible but declining), clinical stage (T2N0 vs T3/T4aN0 vs T1 to 4aN1), and region (United States vs European Union vs most of world).
The primary end point of the study was EFS by blinded independent central review, and key secondary end points were OS and pCR by central pathologist review. Investigators also evaluated safety and EFS by pCR status.
In 2019, the study launched with 2 treatment arms, where patients were randomly assigned 1:1 to receive perioperative pembrolizumab with RC + PLND vs RC + PLND alone. A year later, in 2020, the third treatment arm, perioperative enfortumab vedotin plus pembrolizumab with RC + PLND, was added; patients were then randomly assigned 1:1:1 between the 3 arms. In 2022, investigators expanded the inclusion criteria to include patients who were eligible for cisplatin but refused cisplatin-based treatment. In the same year, they stopped randomly assigning patients to receive pembrolizumab plus RC + PLND and updated the trial enrollment to a 1:1 randomization for the enfortumab vedotin and control arms. They allowed patients in the control arm to receive adjuvant nivolumab (Opdivo) when indicated and available.
The efficacy of enfortumab vedotin plus pembrolizumab and RC + PLND was compared with the control and evaluated in all concurrently randomly assigned patients; these patients comprised the intention-to-treat (ITT) population. Investigators evaluated safety in all patients who had received at least 1 dose of treatment, including surgery. KEYNOTE-905 will continue to examine additional hypotheses for the perioperative pembrolizumab arm, Vulsteke said.
The median patient age was 74.0 years (range, 47-87) in the enfortumab vedotin arm vs 72.5 years (range, 46-87) in the control arm. Most patients were male (80.6% vs 75.3%), had an ECOG performance status of 0 (60.0% vs 54.6%), and were from the European Union (45.9% vs 44.3%) or most of the world (41.8% vs 42.5%). The majority of patients were not eligible for cisplatin (83.5% vs 79.9%), although 16.5% vs 20.1% of patients were eligible but refused cisplatin-based treatment. In the enfortumab vedotin arm, 17.6% of patients had T2N0 (17.6%), T3/T4aN0 (78.2%), and T1 to 4aN1 (4.1%) disease; in the control arm, these rates were 18.4%, 75.9%, and 5.7%, respectively.
In the enfortumab vedotin arm, 167 patients started neoadjuvant treatment, and 144 patients completed it; 149 patients underwent surgery, and 147 patients had complete resection. Those who did not undergo surgery did not because of withdrawal from the trial (n = 10), toxicity (n = 7), disease progression (n = 3), or physician decision (n = 1). A total of 100 patients began the adjuvant phase. In the control arm, 156 patients underwent surgery, and 149 patients had complete resection. In the 18 patients who did not undergo surgery, reasons included withdrawal (n = 13), adverse effect (AE; n = 3), loss to follow-up (n = 1), and physician decision (n = 1).
The median follow-up from randomization to the data cutoff date of June 6, 2025, was 25.6 months (range, 11.8-53.7).
“PFS benefit was consistent across subgroups, including age, ECOG performance status, PD-L1 [expression,] and tumor stage,” Vulsteke said. “The OS benefit was [also] consistent across the subgroups, including age, ECOG performance status, and PD-L1 [expression].”
An exploratory analysis of EFS by pCR status was conducted in the ITT population. In those who received enfortumab vedotin plus pembrolizumab and achieved a pCR (n = 97), the median EFS was NR (95% CI, NR-NR) vs 41.2 months (95% CI, 12.7-NR) in those in the control arm who achieved pCR (n = 15; HR, 0.43; 95% CI, 0.16-1.16). In those in the enfortumab vedotin arm who did not achieve a pCR (n = 73), the median EFS was 26.1 months (95% CI, 10.1-41.2) vs 14.2 months (95% CI, 10.1-19.5) for those in the control arm who did not have a pCR (n = 159; HR, 0.76; 95% CI, 0.51-1.14).
“EFS benefits [were seen with] enfortumab vedotin plus pembrolizumab, irrespective of pCR,” he noted. “But when we look at the pCR, it’s a bad prognostic factor, but also the patients with a pCR in the control arm seem at risk and stay as a high unmet medical need.”
Any-grade treatment-emergent AEs (TEAEs) occurred in all safety-evaluable patients in the enfortumab vedotin arm (n = 167) and 64.8% of those in the control arm (n = 159); these TEAEs were grade 3 or higher for 71.3% and 45.9% of patients, respectively. Serious TEAEs occurred in 58.1% of those in the enfortumab vedotin arm and 40.9% of those in the control arm. AEs led to surgery delay for 4.0% of patients in the enfortumab vedotin arm and 0.6% of those in the control arm. Toxicities led to dose reduction or discontinuation of enfortumab vedotin for 16.8% and 41.3% of patients; they led to discontinuation of pembrolizumab for 34.1% of patients; and they proved fatal for 7.8% of those in the enfortumab vedotin arm and 5.7% of those in the control arm.
The most common TEAEs experienced in all phases of treatment with enfortumab vedotin plus pembrolizumab included pruritus (47.3%), alopecia (34.7%), diarrhea (34.1%), fatigue (32.3%), anemia (30.5%), decreased appetite (28.1%), dysgeusia (28.1%), constipation (27.5%), nausea (25.7%), rash (25.1%), increased aspartate aminotransferase level (24.0%), urinary tract infection (24.0%), weight decrease (19.8%), increased alanine aminotransferase level (19.2%), asthenia (17.4%), maculopapular rash (16.2%), and dry skin (15.0%). During the surgery phase, the most common TEAEs experienced in the enfortumab vedotin arm were anemia (13.7%), prostate cancer (11.6%), and urinary tract infection (8.9%).
Enfortumab vedotin–related AEs of special interest included skin reactions (57.5%), peripheral neuropathy (36.5%), ocular disorders (17.4%), hyperglycemia (9.6%), and infusion-related reactions (1.2%). AEs of special interest associated with pembrolizumab included hypothyroidism (14.4%), severe skin reactions (13.8%), hyperthyroidism (4.8%), pneumonitis (3.6%), hepatitis (3.6%), thyroiditis (3.0%), colitis (2.4%), gastritis (2.4%), nephritis (2.4%), adrenal insufficiency (0.6%), myasthenic syndrome (0.6%), myocarditis (0.6%), and myositis (0.6%).
“The safety profile of perioperative enfortumab vedotin plus pembrolizumab was manageable and consistent with prior reports of this regimen in the locally advanced or metastatic urothelial carcinoma setting,” Vulsteke concluded. “No new safety signals were observed.”
Disclosures: Vulsteke disclosed receipt of research funding and medical writing support from Merck Sharp & Dohme LLC for the present work. He serves on the advisory board for MSD, Janssens-Cilag, GSK, Astellas Pharma, BMS, Leo Pharma, Bayer, AstraZeneca, Pfizer, Merck, and Atheneum Partners. Research grant to the institution was provided by MSD.

By Karen Freifeld
(Reuters) -U.S.-based electronic components distributor Arrow Electronics said on Saturday the U.S. government was reversing trade restrictions placed on Arrow’s China-based affiliates for facilitating the sale of U.S. components found in weaponized drones used by Iran-backed groups like the Houthis.
Arrow (China) Electronics Trading Co and another Arrow entity with six aliases in Hong Kong were added to the Commerce Department’s Entity List on October 8 in a Federal Register posting.
Licenses are required to export goods and technology to companies on the list and are likely to be denied. Firms are placed on the list over U.S. national security or foreign policy interests.
On October 8, Commerce said that drones operated by Iran-backed groups and their debris recovered in the Middle East since 2017 had U.S. components traced to sales tied to these Arrow-related entities.
Arrow said on Saturday the Commerce Department told it the department would soon publish the reversal in the U.S. Federal Register and sent a letter Friday removing the restrictions in the meantime.
“We have received official communication from the U.S. Commerce Department,” Arrow spokesman John Hourigan said in an email. “Arrow is authorized to resume shipping to and from these entities under the same conditions that applied prior to October 8.”
Asked about the matter, a spokesperson for the U.S. Department of Commerce’s Bureau of Industry and Security said in an email: “BIS is committed to ensuring that export restrictions are appropriately targeted to protect national security.”
Hourigan said the company operates in compliance with all laws and regulations. Centennial, Colorado-based Arrow Electronics had global 2024 sales of $28 billion.
Hourigan said that Arrow Electronics (Hong Kong) Co. Ltd, which he described as a subsidiary when it was added to the Entity List, was not actually affiliated with Arrow Electronics.
However, the six aliases tied to the Hong Kong company in the Federal Register posting are affiliated with Arrow and, the company said, would be removed from the Entity List.
(Reporting by Karen Freifeld; Editing by Cynthia Osterman)

On the surface of Mars, there are numerous features that tell of a past age when the planet was warmer and wetter, with rivers, lakes, and even an ocean that covered much of its northern hemisphere. These include river channels, delta…

Nkosi Ndebele won six of seven fights before engaging in a trilogy with Jose Torres, winning two out of the three fights. Ndebele is a former regional bantamweight champion and a striking specialist. Ndebele enters…

Phasmophobia developer Kinetic Games has revealed a new map for the game, set inside an abandoned restaurant. The location–Neil’s Diner–was unveiled during a TwitchCon panel that celebrated the recent fifth anniversary of the…

It’s a busy week for union organisers at Activision Blizzard. Yesterday, in conjunction with the Communications Workers of America (CWA), a group of over 100 developers that work on Hearthstone and the mobile only strategy game…
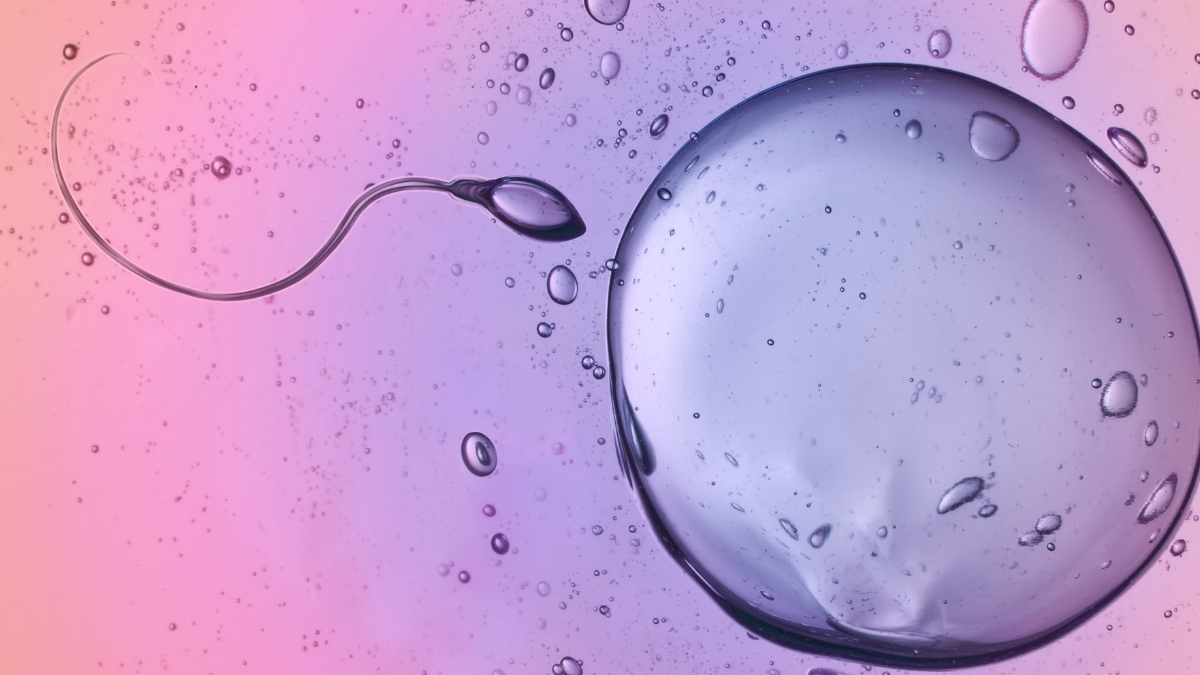
Human sperm can swim through surprisingly viscous fluids with ease – and they seemingly defy Newton’s third law of motion to do so.
To figure out how they slither through substances that should, in theory, resist their movement, a team led…

Neoadjuvant treatment with fam-trastuzumab deruxtecan-nxki (T-DXd; Enhertu) followed by paclitaxel, trastuzumab (Herceptin), and pertuzumab (Perjeta; THP) led to an improvement in pathologic complete response (pCR) rate compared with dose-dense doxorubicin and cyclophosphamide plus THP (ddAC-THP) in patients with high-risk, HER2-positive early breast cancer, according to data from the phase 3 DESTINY-Breast11 trial (NCT05113251).1
Findings presented at the
“DESTINY-Breast11 showed the highest reported pCR rate in HER2-positive early breast cancer for a registrational study in the neoadjuvant setting, despite—if you want to do cross-trial comparisons—a high prevalence of hormone receptor–positive disease and a high-risk population,” lead study author Nadia Harbeck, MD, PhD, said in a presentation of the data.
Harbeck is director of the Breast Center and chair for Conservative Oncology at the Department of OB&GYN at LMU University Hospital in Munich, Germany.
Harbeck noted that current neoadjuvant standard-of-care (SOC) regimens for HER2-positive early breast cancer have remained unchanged for more than a decade. As such, investigators sought to evaluate T-DXd–based treatment in this population with the goal of improving efficacy and safety vs the current SOC.
The randomized, global, multicenter, open-label study enrolled patients with previously untreated HER2-positive early breast cancer who had high-risk disease, defined as ≥cT3 and N0-3 or cT0-4 and N1-3; or inflammatory breast cancer. Patients were allowed to enroll, irrespective of hormone receptor status.
Patients were randomly assigned in a 1:1:1 fashion to receive T-DXd followed by THP; ddAC-THP; or T-DXd alone, followed by surgery in all arms. In the first arm, patients received 4 cycles of T-DXd followed by 4 cycles of THP. In the second, ddAC was administered for 4 cycles, followed by 4 cycles of THP. In the final arm, patients received T-DXd alone for 8 cycles.
Notably, the T-DXd monotherapy arm was closed in March 2024, following a recommendation from the study’s independent data monitoring committee.
After surgery, study protocols called for the following treatments, irrespective of arm:
The study’s primary end point was pCR rate (ypT0/Tis ypN0) per blinded independent central review (BICR) assessment. Secondary end points included BICR-assessed pCR rate (ypT0 ypN0), event-free survival (EFS), safety, pharmacokinetics, invasive disease-free survival, overall survival, and health-related quality of life. Residual cancer burden (RCB) was an additional outcome measured during the study.
At data cutoff, 16.9% of patients in the T-DXd plus THP arm discontinued a study drug, including T-DXd (2.8%), paclitaxel (14.4%), trastuzumab (2.2%), and pertuzumab (2.2%); 97.2% of patients in this arm proceeded to surgery. In the ddAC-THP arm, 13.8% of patients had discontinued any study treatment, including AC (2.9%), paclitaxel (12.0%), trastuzumab (3.0%), and pertuzumab (3.7%); 93.8% of patients in this arm underwent surgery. In the T-DXd monotherapy arm (n = 286), 18.4% of patients discontinued study treatment, and 95.8% underwent surgery.
Regarding post-neoadjuvant treatments, 99.1% of evaluable patients in the T-DXd arm who achieved a pCR (n = 226) underwent any adjuvant therapy, comprising any cytotoxic chemotherapy regimen (5.8%), any T-DM1–containing regimen (1.8%), and any trastuzumab-containing regimen (94.2%). In the ddAC-THP, 98.4% of patients who achieved a pCR (n = 190) underwent any adjuvant therapy, including any cytotoxic chemotherapy regimen (5.8%), any T-DM1–containing regimen (2.1%), and any trastuzumab-containing regimen (91.6%).
For patients who did not achieve a pCR, any adjuvant therapy was administered to 89.5% of patients in the T-DXd plus THP arm (n = 95) and 82.3% of patients in the ddAC-THP arm (n = 130). In the experimental group, adjuvant therapies included any cytotoxic chemotherapy regimen (10.5%), any T-DM1–containing regimen (52.6%), and any trastuzumab-containing regimen (38.9%). These respective rates were 9.2%, 56.9%, and 26.2% in the control group.
In the T-DXd plus THP and ddAC-THP arms, the median age was 50 years (range, 25-82) and 50 years (range, 23-79), respectively. All patients in both arms were female. The highest proportion of patients in each arm was from Asia (T-DXd plus THP, 47.4%; ddAC-THP, 47.5%) and were Asian (49.8%; 49.1%). Most patients had an ECOG performance status of 0 (86.6%; 87.5%), had immunohistochemistry 3+ HER2-positive disease (87.2%; 88.4%), had cT0-2 tumors (54.8%; 58.8%), and had positive lymph nodes (89.4%; 87.8%).
Findings also showed that 81.3% of patients in the T-DXd plus THP arm had no RCB (RCB-0) or minimal RCB (RCB-1) in resected breast or lymph node tissue compared with 69.1% in the ddAC-THP arm (difference, 12.2%). In the hormone receptor–positive population, the RCB-0 plus RCB-1 rates were 78.0% for T-DXd plus THP vs 64.7% for ddAC-THP; these respective rates were 90.4% and 81.2% in the hormone receptor–negative population.
Investigators also reported an EFS trend favoring T-DXd plus THP, with data at 4.5% maturity (HR, 0.56; 95% CI, 0.26-1.17). Maturity for the data cutoff of the trial’s final analysis is predicted at approximately 10%. The 24-month EFS rates were 96.9% (95% CI, 93.5%-98.6%) in the T-DXd plus THP arm vs 93.1% (95% CI, 88.7%-95.8%) in the ddAC-THP arm.
Any-grade adverse effects (AEs) occurred in 98.1% of patients in the T-DXd plus THP arm compared with 98.7% of patients in the ddAC-THP arm. The respective rates of grade 3 or higher AEs were 37.5% and 55.8%. Any-grade serious AEs were reported in 10.6% and 20.2% of patients, respectively.
In the T-DXd plus THP arm, AEs led to dose any dose reduction, any drug interruption, and any treatment discontinuation in 18.1%, 37.8%, and 14.1% of patients, respectively. In the ddAC-THP group, these rates were 19.2%, 54.5%, and 9.9%, respectively. AEs led to death in 0.6% of patients in both arms. AEs led to delays in surgery in 3.4% of patients in the experimental arm vs 2.6% of patients in the control arm.
Regarding AEs of special interest, any-grade drug-related adjudicated interstitial lung disease (ILD) was 4.4% in the T-DXd plus THP arm vs 5.1% in the ddAC-THP arm. The rates of grade 3 or higher ILD were 0.6% and 1.9%, respectively. Grade 5 ILD occurred in 1 patient (0.3%) in both groups. Any-grade left ventricular dysfunction occurred in 1.3% of patients in the experimental arm vs 6.1% of patients in the control arm. The rates of grade 3 or higher left ventricular dysfunction were 0.3% and 1.9%, respectively, although no grade 5 events were reported in either group.
Any-grade treatment-emergent AEs reported in at least 20% of patients in either arm included nausea (T-DXd plus THP, 64.7%; ddAC-THP, 51.6%), diarrhea (58.8%; 54.2%), alopecia (47.5%; 49.0%), fatigue (41.3%; 54.8%), increased transaminase levels (34.4%; 33.7%), neutropenia (29.1%; 44.2%), constipation (29.1%; 24.4%), vomiting (28.8%; 21.2%), peripheral neuropathy (25.9%; 20.8%), anemia (22.8%; 49.7%), stomatitis (18.4%; 27.9%), and leukopenia (17.2%; 23.4%).
When the T-DXd monotherapy arm, patients were allowed to remain on therapy or immediately switch to local SOC. If patients switched therapy, they were classified as having a non-pCR.
Findings from the monotherapy arm showed that patients (n = 286) achieved a pCR rate of 43.0% at the primary analysis and 51.4% at a prespecified supplementary analysis. EFS data were similar between T-DXd monotherapy and ddAC-THP (HR, 0.82; 95% CI, 0.41-1.62), and the 24-month EFS rate in the T-DXd monotherapy group was 94.4% (95% CI, 90.5%-98.7%).
Regarding safety, 97.5% of patients treated with T-DXd alone (n = 283) experienced any-grade AEs, 22.6% had grade 3 or higher AEs, and 10.2% had any-grade serious AEs. AEs led to dose reduction, drug interruption, and treatment discontinuation in 6.7%, 18.0%, and 7.8% of patients, respectively. One patient (0.4%) experienced an AE that led to death. AEs led to surgical delay in 6.4% of patients.
The rate of any-grade, drug-related adjudicated ILD was 4.9% in the T-DXd monotherapy arm, although all instances were grade 1 or 2. Left ventricular dysfunction occurred in 0.7% of patients, all at grade 1 or 2.
Based on data from DESTINY-Breast11, the FDA accepted a supplemental biologics license application seeking the approval of neoadjuvant T-DXd followed THP for the treatment of adult patients with high-risk, HER2-positive (IHC 3+ or in situ hybridization–positive), stage II/III breast cancer.2
The FDA has assigned a target action date of May 18, 2026, under the Prescription Drug User Fee Act.
“DESTINY-Breast11 results support T-DXd [plus] THP as a more effective and less toxic neoadjuvant treatment compared with ddAC-THP, and it may become the preferred regimen for patients with high-risk, HER2-positive early breast cancer,” Harbeck concluded in her presentation.1
Disclosures: Harbeck reported receiving honoraria from AstraZeneca, Daiichi Sankyo, Gilead, Lilly, Menarini Stemline, MSD, Novartis, Pfizer, Pierre Fabre, Roche, Viatris, and Zuellig Pharma; serving as a consultant or advisor for Exact Sciences, Gilead, Pfizer, Roche, and Sandoz; having an institutional site contract with AstraZeneca; serving as a data safety monitoring board or advisory board member for Gilead, IQVIA, and Roche; and having ownership interested in the West German Study Group.

I love the 30 Days of Night franchise, so I was super excited to discover this week that it’s picking back up with Falling Sun, and this first issue did not disappoint. 30 Days of Night: Falling Sun is written by Rodney Barnes (Killadelphia), who…