Bitcoin trades near $121.6K, testing major resistance at $126K. Analysts watch for a breakout or correction as futures open interest stays high.
Bitcoin is trading near $121,600 after reaching a new all-time high above $126,000 earlier this week. The asset has slowed and is now moving sideways just below a major diagonal resistance that has capped the price for several months. Analysts are watching to see whether Bitcoin can break through or if it will be rejected once again.
Meanwhile, the market is holding near a key level while open interest remains high in the futures market.
Bitcoin Struggles Near Long-Term Resistance
Bitcoin is facing resistance at a trendline that has held firm since earlier cycle tops. The latest weekly candle shows another rejection near $126,000, followed by a pullback of around 5.5%. Earlier this year, similar rejections led to drops of nearly 10% and close to 30%.
The difference this time is the size of the pullbacks. Each rejection has been smaller than the last. Rekt Capital pointed out that “this trendline looks to be a weakening point of rejection,” suggesting sellers may be losing strength at this level.
On the lower timeframes, Bitcoin is holding just above $121,000. This zone is marked by a previous support level and lines up with a Fibonacci retracement near $119,550. Daan Crypto Trades noted that this is the area to watch.
$BTC Not here to overcomplicate things.
We saw a sharp impulse up and an all time high sweep.
Currently price is consolidating beneath this diagonal resistance. If that’s broken, I am expecting this to move to new highs pretty quickly.
Key area to hold below is ~$120K. If… pic.twitter.com/MK7gYzINWJ
— Daan Crypto Trades (@DaanCrypto) October 10, 2025
The asset is currently consolidating in a tight range. Volume has slowed, which often happens before a larger move. The trend remains bullish overall. A confirmed higher low would support another leg higher, especially if resistance breaks.
You may also like:
Bearish Scenario Still in Play
While short-term sentiment remains positive, some market participants are still cautious. Analyst Ali Martinez shared a scenario where Bitcoin gets rejected at $124,000 and begins a correction toward $96,000. If that level fails, the next major support is around $70,000.
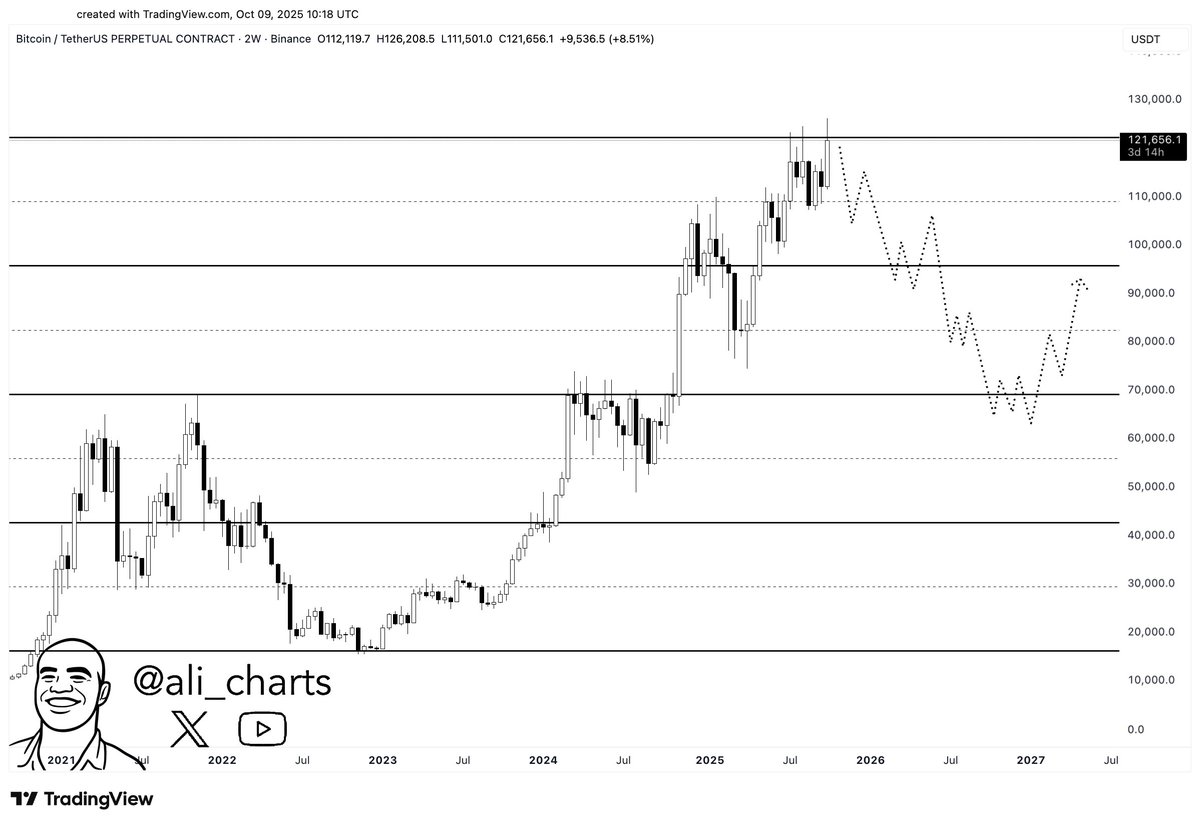
The projected path shows a slow recovery beginning in late 2026, with Bitcoin climbing back toward $90,000. This scenario reflects a broader retracement, not a trend reversal, and suggests long-term structure could still remain intact.
Futures Market Remains Heated
Data from Glassnode shows that futures open interest is still elevated. This means that many traders are positioned on both the long and short sides. Sharp moves in either direction have triggered liquidations. Glassnode said the market is undergoing “a leverage reset” as volatility flushes out excess positions.
With Bitcoin sitting just below resistance and leverage still high, traders are watching closely. A move above the current range could open the way toward $130,000. If resistance holds, another pullback could extend the current consolidation.
Binance Free $600 (CryptoPotato Exclusive): Use this link to register a new account and receive $600 exclusive welcome offer on Binance (full details).
LIMITED OFFER for CryptoPotato readers at Bybit: Use this link to register and open a $500 FREE position on any coin!