When actor Steve Evets was offered the role of foul-mouthed farmer Jim on a new Sky series, it was as easy yes.
“It’s a dream job,” says Evets, who has starred in films and TV series including Looking for Eric, One for Us and White Gold.
“They…

When actor Steve Evets was offered the role of foul-mouthed farmer Jim on a new Sky series, it was as easy yes.
“It’s a dream job,” says Evets, who has starred in films and TV series including Looking for Eric, One for Us and White Gold.
“They…
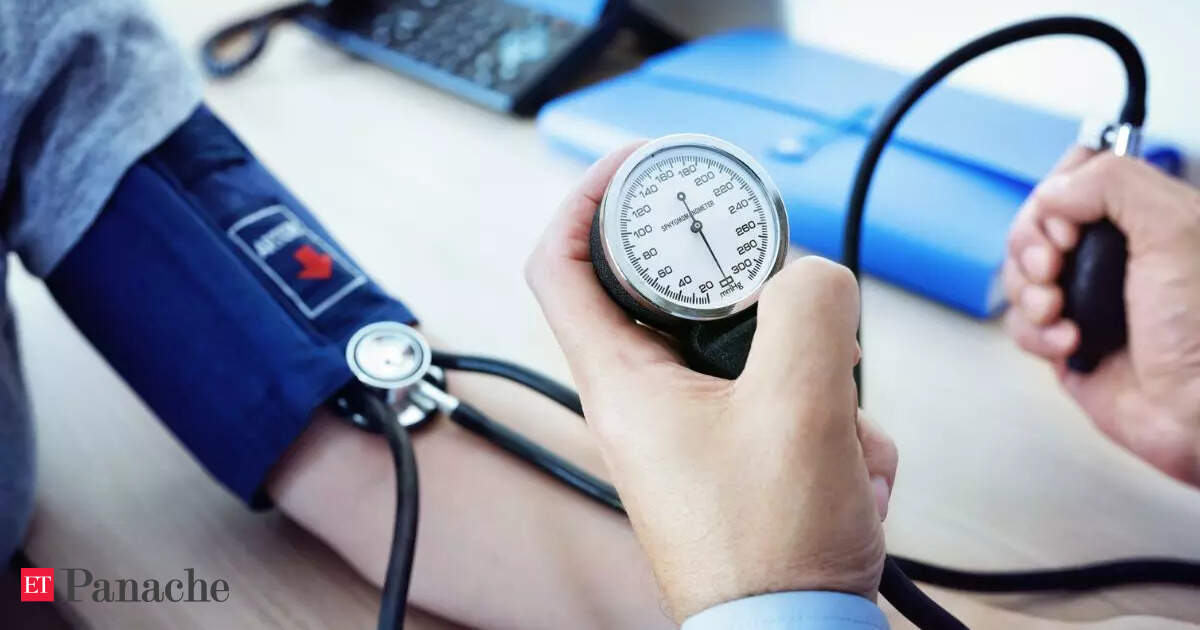

The phase 3 ASTRUM-006 trial examining serplulimab (Hansizhuang) plus chemotherapy as neoadjuvant/adjuvant monotherapy treatment in patients with gastric cancer met its primary end point of event-free survival (EFS), according to an announcement from Shanghai Henlius Biotech, Inc.1
Additional data from the interim analysis conducted by an independent data monitoring committee (IDMC) showed that serplulimab plus chemotherapy elicited a pathologic complete response (pCR) rate that was threefold higher than that achieved with placebo plus chemotherapy and also significantly reduced the risk of recurrence. The combination was noted to have an acceptable toxicity profile, with no new safety signals reported.
Based on these findings, the IDMC has recommended early submission of a new drug application for serplulimab.
“Surgery is the cornerstone of gastric cancer treatment, and perioperative therapy is critical to long-term survival,” Professor Jiafu Ji, of Beijing Cancer Hospital, stated in a news release. “This study is the first to confirm the feasibility of replacing adjuvant chemotherapy with mono-immunotherapy in the postoperative setting. It not only opens a new path to consolidate surgical outcomes and reduce recurrence risk but also paves the way for innovation in clinical practice.”
Data from a study shared at the
The treatment-related adverse effects (TRAEs) that were most commonly experienced with the regimen were nausea, anorexia, thrombocytopenia, fatigue, and thyroid dysfunction. No TRAEs were grade 3 or higher in severity. The study authors concluded that the findings supported a place for immune-based neoadjuvant therapy in this setting.
The randomized, double-blind, phase 3 ASTRUM-005 study (NCT04063163) randomized patients with extensive-stage small cell lung cancer to serplulimab at 4.5 mg/kg on day 1 plus carboplatin at an area under the curve of 5 on day 1 and 100 mg/m2 of etoposide on days 1 to 3 Q3W for up to 4 cycles followed by maintenance serplulimab at 4.5 mg/kg Q3W or placebo plus the same chemotherapy regimen.3
Data shared during the 2025 ASCO Annual Meeting showed that those who received serplulimab (n = 389) experienced a median OS of 15.8 months (95% CI, 13.9-17.4) compared with 11.1 months (95% CI, 10.0-12.4) with placebo (n = 196), translating to a 40% reduction in the risk of death (HR, 0.60; 95% CI, 0.49-0.73; descriptive P < .001). The OS rates in the respective arms at 4 years were 21.9% (95% CI, 17.6%-26.6%) and 7.2% (95% CI, 3.8%-12.1%). The median progression-free survival with serplulimab was 5.8 months (95% CI, 5.6-6.9) vs 4.3 months (95% CI, 4.2-4.4), translating to a 53% reduction in the risk of disease progression or death (HR, 0.47; 95% CI, 0.38-0.57; descriptive P < .001).
Serplulimab plus chemotherapy elicited a confirmed objective response rate (ORR) of 68.9% (95% CI, 64.0%-73.5%), with a median duration of response (DOR) of 6.8 months (95% CI, 5.5-7.9). In the placebo arm, the confirmed ORR was 58.7% (95% CI, 51.4%-65.6%) and the median DOR was 4.2 months (95% CI, 3.1-4.2; HR for DOR was 0.45; 95% CI, 0.35-0.58; descriptive P < .001).
In June 2025, the Medicines and Healthcare Products Regulatory Agency of the
Serplulimab plus carboplatin and nab-paclitaxel (Abraxane) is also being evaluated in patients with previously untreated locally advanced or metastatic squamous non–small cell lung cancer. Data from the final analysis of the

 Getty Images
Getty ImagesAuthor Peter James has revealed he was contacted by Queen Camilla to ask if she could be a central character in his upcoming novel.
James, who…

An immigrant drama by Rudi Goblen about two brothers born in Nicaragua, “littleboy/littleman,” now receiving its world premiere at the Geffen Playhouse, is an American story at its core.
Lest we forget our past, America is the great…

“She wrote to me and asked when will a novel be set in London”, James said, adding that things had not previously ended well for those who previously ignored Queen’s of England.
“I was aware that there was chaos in the palace due to renovations,…

After being recognised at the Mobile Photography awards 2020, Christian Barroso travelled from his home in Rio de Janeiro to São Paulo for the exhibition of winning images. He was joined by three friends and, after seeing his work on display,…

The planets around white dwarf stars might provide long-term homes for alien life, but they suffer from a fatal overheating problem. Who’s going to rescue them? According to new research, it’s none other than Albert Einstein.
White dwarfs are the…