Fact File: Interstellar Comet 3I/ATLAS
Type: Interstellar comet (third confirmed visitor from beyond our solar system)
Origin: Likely ejected from a distant star system millions of years ago
Path: “Hyperbolic” trajectory — one-time pass through…

Type: Interstellar comet (third confirmed visitor from beyond our solar system)
Origin: Likely ejected from a distant star system millions of years ago
Path: “Hyperbolic” trajectory — one-time pass through…

The Community Studio, located on the first and second floors, welcomes guests with an expansive and interactive Auto Library featuring over 2,500 curated books and 500 mobility-related lifestyle and exhibition items. As a collaboration between Hyundai Motor, CCC (Culture Convenience Club), the lifestyle company behind TSUTAYA BOOKSTORE, French luxury publisher ‘Assouline’, and Korean mobility culture company ‘EREVO’, the Auto Library is a must for all those who want a deeper insight into automotive milestones. Visitors can browse through vintage catalogs, admire collectible model cars, read contemporary automotive lifestyle articles, and view racing goods, along with a variety of Hyundai memorabilia.
Rare items such as the Camel Trophy archive—one of only five limited sets worldwide are displayed among other, carefully sourced volumes that narrate the backstories, personal histories, people, and diverse mobility brands shaping modern car culture.
Once a simple text editor, Microsoft’s Visual Studio Code has transformed into a community-driven open source platform under the MIT license.
Visual Studio…

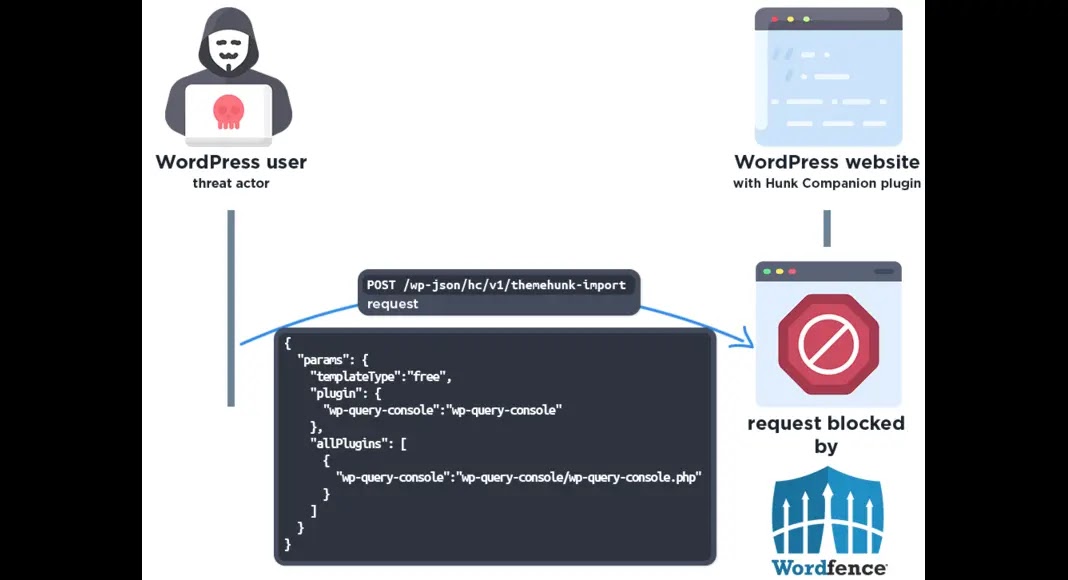
Critical vulnerabilities in two popular WordPress plugins are under active mass exploitation, with Wordfence blocking over 8.7 million attack attempts targeting arbitrary plugin installation flaws.
The security firm warns that threat actors…

TOKYO — Japanese sushi legend Jiro Ono won three Michelin stars for more than a decade, the world’s oldest head chef to do so. He has served the world’s dignitaries and his art of sushi was featured in an award-winning film.
After all these…

It is common knowledge that tuberculosis is one of the most serious public health problems worldwide. According to the World Health Organization’s (WHO) Global Tuberculosis Report 2024 it is estimated that there are approximately…

Teicoplanin (TEC), a glycopeptide antibiotic, is widely used for treating invasive infections caused by Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA), methicillin-sensitive Staphylococcus aureus (MSSA), Streptococcus pneumoniae, and Enterococcus species.1 Its mechanism of action involves binding to the D-alanyl-D-alanine termini of peptidoglycan precursors, inhibiting bacterial cell wall synthesis.2 Due to its broad-spectrum activity and favorable safety profile, TEC is commonly administered for pediatric infections such as bloodstream infections, endocarditis, and osteomyelitis.3
Given its poor oral bioavailability, TEC is typically administered intravenously or intramuscularly in pediatric patients. The drug exhibits a long half-life, enabling less frequent dosing; however, its high protein-binding capacity (90–95%) may influence distribution and elimination.4 The weight-normalized clearance (CL) of TEC in children (0.21–1.99 L/h/70 kg) is generally higher than that in adults (0.45–6.33 L/h/70 kg, though mostly concentrated at 0.5–1.0 L/h/70 kg). Additionally, the CL of TEC in children is more significantly influenced by age (eg, differences between infants and older children), body weight, and renal function, whereas in adults, it is mainly associated with renal function. Although TEC exhibits linear pharmacokinetics (PK) within the therapeutic concentration range, pediatric patients face unique PK challenges due to developmental changes in renal/hepatic function, body composition, and drug metabolism.5 These factors contribute to significant interindividual variability in drug exposure, necessitating tailored dosing strategies to avoid subtherapeutic concentrations or toxicity.
The clinical efficacy of TEC is closely associated with pharmacokinetics/pharmacodynamics (PK/PD) indices, particularly the ratio of the area under the concentration–time curve to the minimum inhibitory concentration (AUC24/MIC).6 The trough concentration (Cmin) represents another critical efficacy parameter, with Cmin >10 mg/L demonstrating improved clinical outcomes in invasive infections.7–9 However, achieving optimal PK/PD targets in pediatric populations remains challenging due to limited exposure-response data and the impact of covariates such as age, weight, and renal function.10
Treatment failure in pediatric patients may result from bacterial resistance, inadequate dosing, or variability in drug exposure.11 Population pharmacokinetic (PPK) modeling provides a robust approach to identify covariates influencing TEC pharmacokinetics and optimize dosing regimens.12 Despite its clinical significance, no systematic review had comprehensively evaluated PPK studies of TEC in pediatric populations. Therefore, this review aims to: (1) summarize significant predictors of teicoplanin PK parameters; (2) analyze the probability of target attainment (PTA) for primary PK/PD indices across pediatric subgroups using model-based simulations; and (3) identify knowledge gaps requiring further investigation.
A systematic search was conducted for all PPK studies of TEC in the PubMed, Web of Science, and EMBASE databases from their inception until 25 March 2025. The search terms used were: “Teicoplanin”, “Targocid”, “Teichomycin”, “Teichomycin A2.” Additionally, we included terms related to pharmacokinetics such as “population pharmacokinetic*” “pharmacokinetic model*” “nonlinear mixed-effects model”, “NONMEM”, “WINNONMIX”, “P-PHARM”, “MWPHARM”, “nlmixed”, “NLME”, or “MONOLIX.” Finally, we searched for terms related to age groups: “Children”, “Child”, “Neonate*” “Neonatal”, “Infant*” “Newborn”, and “Pediatric*.” The reference lists of all included studies were manually checked to retrieve potentially relevant studies. Detailed search strategies were listed in Supplementary Table S1.
A study was deemed eligible if it met the following criteria: (1) the study population consisted of humans, (2) TEC was the study drug, (3) a population-based analysis was employed, and (4) a nonlinear mixed-effect modeling approach was adopted. A study was excluded if (1) it was a review, conference abstract, or focused on methodology/algorithm/software, (2) it was published in a non-English language, (3) its data overlapped with an article published later, and (4) the information on modeling was insufficient to reproduce.
Moreover, two investigators independently conducted the literature search and study selection process. For discrepancies, consensus was achieved through consultation with a senior researcher. The systematic review methodology adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.13
The following information was extracted from the included articles: (1) the characteristics of the target population (eg age, weight range, and sex); (2) the study design (eg type of study, number of participants and collected samples, sampling design, and TEC formulations); and (3) the information on PPK analyses such as data analysis software, structural models, between-subject variability (BSV), residual unexplained variability (RUV), parameter estimates, covariates, and model evaluation approaches. The study characteristics and PPK analyses were summarized in a tabular format.
The reporting quality of the included studies was assessed based on the reporting guidelines for PK studies.14,15 A checklist containing 35 items was created to summarize the key points of the PK report (Supplementary Table S2). All items were categorized into five sections: title/abstract, background, methods, results, and discussion/conclusion. If an item in the checklist was reported in the study, one point was assigned; otherwise, no point was counted. The compliance rate was used to evaluate the quality of each study, and the calculation formula was as follows (Equation 1):
Compliance rate (%) = sum of items reported / sum of all items × 100% (1)
Monte Carlo simulations of PK profiles were performed to provide visual predictive distributions (VPDs) based on the established model and the study cohort in each included study.16 According to the classifications of patient characteristics in the retrieved studies,17–24 we adapted the following 5 groups of virtual populations for Monte Carlo simulation: preterm infants, neonates, infants, children, and adolescents. Differences in each profile were compared by visual inspection because it was assumed that the predictive distribution of the simulated PK profile of TEC sufficiently represented the features of each model and its original data.16 A total of 1000 virtual patients were simulated for each scenario. All simulations were performed using NONMEM (version 7.4.1; ICON Development Solutions, Ellicott City, MD, U.S.A). R software (version 4.3.1; www.r-project.org, accessed on 16 June 2023) was employed for data exploration and visualization.
Clearance (CL) is the most critical PK parameter in long-term pharmacotherapy and has been comprehensively investigated in previous studies. Therefore, the covariate effect on CL was explored. The effects of the included covariates on PK parameters were assessed using forest plots. For continuous covariates, the maximum and minimum values based on the demographic information in the included studies were extracted and scaled to the same range. For binary covariates such as sex, 0 and 1 were used.
Based on the range of the identified covariate in each study, the minimum and maximum CL values were calculated. The CL value, normalized to median covariate values in each study, was selected as the reference. Thus, the effect of the identified covariate on CL in each study was displayed by the following equation (Equation 2):
Effect of covariatej in studyi = The range of calculated CL / The CL reference in studyi × 100% (2)
where studyi means the ith study and covariatej means the jth identified covariates in studyi.
Moreover, the change in CL within 80%–125%, a threshold widely used to assess bioequivalence,25 was not considered clinically significant in our study. All data were analyzed and plotted using R software (version 4.3.1; www.r-project.org, accessed on 16 June 2023).
AUC24/MIC ≥ 400 and Cmin > 10 mg/L are associated with effective bacterial suppression and reduced development of antimicrobial resistance in the treatment of MRSA infections.19–21,26 Based on the EUCAST standard, the MIC breakpoint for susceptibility against MRSA is defined as 2 mg/L.27 These values are crucial for guiding the rational use of TEC in clinical practice, facilitating the development of appropriate dosing regimens and the accurate assessment of bacterial resistance.
Monte Carlo simulations were conducted to evaluate the rationality of a consistent dosing regimen across five age groups: preterm infants, neonates, infants, children, and adolescents. The dosing protocol included a loading dose of 10 mg/kg every 12 hours for three doses, followed by a maintenance dose of 10 mg/kg once daily. For each age group, 1,000 virtual patients were simulated. Monte Carlo modeling provided the PK profiles used to determine AUC24 on day 4 by trapezoidal rule integration and Cmin as trough concentrations.
Separate PTA calculations for AUC24/MIC ≥400 and Cmin >10 mg/L were performed across pediatric age groups, with comparative analyses conducted for each target. We set the target that the PTA should be ≥ 90% for determining the optimal dosage for MRSA.
Figure 1 presents the PRISMA flowchart of study selection. A total of 148 records were initially identified through database searches (PubMed: n=39; Embase: n=47; Web of Science: n=62). After duplicate removal (n=64) and automated screening exclusion, 84 records underwent title/abstract screening. Following exclusion of irrelevant studies (n=44), in vitro/animal studies (n=2), non-PPK studies (n=18), reviews (n=2), and conference abstracts (n=1), 17 full-text articles were assessed. Six studies employing adult population models and three utilizing nonparametric approaches were subsequently excluded, yielding eight eligible studies for final inclusion.
|
Figure 1 PRISMA flow diagram used to identify teicoplanin (TEC) population pharmacokinetics studies.
|
The reporting quality assessment of included studies is summarized in Table 1. The median compliance rate was 84.3% (range: 82.9–94.3%), demonstrating generally satisfactory reporting quality. However, several reporting deficiencies were identified: only 37.5% of studies adequately described co-administration or food-related aspects, and 62.5% failed to report methods for handling missing data. Notably, base model evaluation methods were universally unreported, while only 62.5% and 37.5% of studies provided schematic representations of the final model and summaries of model-building processes, respectively. Despite these limitations, all studies maintained compliance rates exceeding 80%, indicating overall methodological robustness.
 |
Table 1 Reporting Quality of Included Studies
|
All included studies were single-center investigations, with characteristics detailed in Table 2. Only Yamada et al23 and Zhang et al20 employed retrospective designs, while the remaining studies17–19,21,22,24 were prospective. The studies encompassed diverse pediatric populations: Lukas et al17 stratified participants by a 12-month age threshold; Zhao et al18 Zhang et al,20 and Aulin et al22 examined broader age ranges; Gao et al19 focused on younger subjects (median age: 1.25 years); Kontou et al21 specifically studied preterm infants and neonates (age range: 6–17.7 days); Yamada et al23 differentiated neonates, infants, and children; and Laiseca et al24 categorized patients by continuous kidney replacement therapy status.
 |
Table 2 Characteristics of Included Studies
|
Quantification methods included high-performance liquid chromatography (HPLC), liquid chromatography-tandem mass spectrometry (LC-MS/MS), ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS), reverse-phase HPLC (RP-HPLC), fluorescence polarization immunoassay (FPIA), quantitative microsphere system (QMS), and latex turbidimetric immunoassay (LTIA). The lower limit of quantification (LLOQ) varied widely, ranging from 25 ng/mL to 3 mg/L.
A total of eight PPK models were identified across the included studies. The majority (7/8)17,18,20–24 employed NONMEM software, with one study using NLME.19 Structural models (7/8) predominantly followed a two-compartment disposition, while one study20 adopted a one-compartment approach. Parameter estimation primarily utilized first-order conditional estimation (FOCE), with or without η-ε interaction. Sampling strategies varied, with two studies22,24 employing intensive sampling and the remaining17–21,23 relying on sparse data from therapeutic drug monitoring (TDM). Study sample sizes ranged from 20 to 214 participants, with total TEC observations per study spanning 143–399. Detailed modeling approaches and final PK parameters are summarized in Table 3.
 |
Table 3 Modeling Strategies and Final Parameters of Included Studies
|
Virtual patients spanning from preterm infants to adolescents were stratified into five demographic groups for VPDs. All simulated cohorts comprised male subjects receiving TEC at steady-state conditions. Population characteristics for five representative groups were detailed in Supplementary Table S3. Monte Carlo simulations generated 1,000 virtual patients per cohort, with results visualized in Figure 2.
 |
Figure 2 Concentration–time profiles of TEC in (A) preterm infants, (B) neonates, (C) infants, (D) children, (E) adolescents in retrieved studies. The solid line represents the median of the simulated concentration–time profile, and the light shadows represent the 10th–90th percentiles of those profiles. All patients were assumed to be male and received TEC at a 10 mg/kg weight-normalized dosage.
|
At therapeutic doses, adolescents exhibited lower median weight-normalized CL values compared to other populations, while neonates showed higher median CL values than infants and children. The estimated median CL of TEC varied by age group: 0.0131 L/h/kg for preterm infants (1.5 kg, 10 days), 0.0150 L/h/kg (range: 0.0114–0.0185) for neonates (3.5 kg, 10 days), 0.0136 L/h/kg (range: 0.0110–0.0488) for infants (7 kg, 0.5 years), 0.0138 L/h/kg (range: 0.00946–0.0370) for children (21 kg, 6 years), and 0.0116 L/h/kg (range: 0.00855–0.0285) for adolescents (60 kg, 14 years). Additionally, no significant population differences were found in the median volume of distribution (V) values of TEC. The median values of V per kilogram (L/kg) were similar for neonates, infants, and children, though higher than those for preterm infants. Specifically, the median values were: 0.159 L/kg for preterm infants, 0.208 L/kg (range: 0.159–0.257) for neonates, 0.248 L/kg (range: 0.0871–0.476) for infants, 0.186 L/kg (range: 0.0871–0.476) for children, and 0.171 L/kg (range: 0.0871–0.476) for adolescents.
The steady-state AUC24 at day 4 (72–96 h post-dose) across age groups is presented in Figure 3. Preterm infants demonstrated the highest median AUC24 (740 mg·h/L), exceeding the 540–690 mg·h/L range observed in neonates. Infants showed substantial between-study variability (median AUC24 range: 206–715 mg·h/L), while children exhibited similar heterogeneity (273–835 mg·h/L). Adolescents displayed the most pronounced variability (350–1110 mg·h/L).
 |
Figure 3 TEC main pharmacokinetic parameter (AUC) at steady state for various typical populations. All simulated patients were assumed to be male. They received TEC with an initial regimen of 10 mg/kg administered every 12 hours for three doses, followed by a maintenance dose of 10 mg/kg once every 24 hours. Notes: The text in different colors in the figure corresponds to each population pharmacokinetic (PPK) study included in this research, with each color uniquely identifying one of the eight studies.
|
All tested covariates that affected CL, and the distribution volume of the central compartment (Vc) are summarized in Table 4. The stepwise covariate screening included forward inclusion and backward elimination. A comparison of identified and investigated covariates is presented in Figure 4.
 |
Table 4 List of Tested and Significant Covariates in the Models
|
 |
Figure 4 A histogram of the amount of investigated and identified covariates in included studies.
|
All included PPK studies of TEC aimed to identify potential covariates explaining between-subject variability (BSV) in drug exposure. The most frequently screened covariates were: weight (investigated in 7 studies), age (6 studies), serum creatinine (Scr; 6 studies), estimated glomerular filtration rate (eGFR; 4 studies), creatinine clearance (CLcr; 3 studies), albumin (ALB; 3 studies), body surface area (BSA; 3 studies), and sex (3 studies). Among these, weight was consistently identified as a significant covariate in all 7 studies that examined it. Other covariates showing statistical significance in final models included: CLcr (2/3 studies), eGFR (2/4 studies), Scr (2/6 studies), postmenstrual age (PMA; 1/1 study), postnatal age (PNA; 1/2 studies), filter surface area (FSA; 1/1 study), age (1/6 studies), ALB (1/3 studies), and continuous kidney replacement therapy (CKRT; 1/1 study). Notably, despite being frequently investigated, covariates such as sex and BSA were not retained in any final models, possibly due to collinearity with stronger predictors or limited sample sizes in individual studies.
The effect of identified covariates on CL was presented in the forest plot (Figure 5). Seven of the 8 studies indicated that body weight was associated with the CL of TEC.18–24 In contrast, one study17 found no relationship between body weight and CL, but it did find an association between weight and CL when conducting population-wide modeling. Weight significantly affected CL changes across all age groups. One study23 revealed that PMA significantly influenced the CL of TEC in a threshold-dependent manner: for patients with PMA ≥48 weeks, eGFR was the primary covariate affecting CL; whereas in those with PMA <48 weeks, CL was determined by PMA itself, PNA, Scr, and ALB. Moreover, the eGFR could explain the BSV of CL in two studies,19,23 all of which showed a clinically significant influence on CL, with absolute changes exceeding 20%.
 |
Figure 5 Covariate effect on the clearance (CL) of TEC. The horizontal bars represent the effect of covariates on the CL according to each study. The typical CL in each study was considered to be 1. The x-axis refers to the effect of each identified covariate on CL, expressed as the ratio of the value of CL in the range of each covariate to the typical value of CL. The shaded area ranges from 0.8 to 1.25. N, no; Y, yes.
|
Two studies18,21 reported CLcr significantly influenced TEC clearance. One study18 demonstrated that when CLcr ranged from 25 to 464.1 mL/min, CL varied by 0.30-to 1.78-fold relative to baseline. The other study,21 for the same range of CLcr values, showed a much larger variation, from 0.99 to 7.04 times. Two independent studies20,23 identified Scr as a significant CL determinant. ALB showed particular clinical relevance for CL in neonates and infants with PMA < 48 weeks.23 One study24 revealed CKRT reduced the CL of TEC by approximately 30% compared to non-CKRT patients. Notably, filter surface area significantly affected CL in CKRT patients: medium and large filters increased CL by 2.58-fold and 4.04-fold, respectively.
All included studies characterized between-subject variability (BSV) using exponential models. The median BSV was 33.1% (range: 14.1%–65.9%; n=8) for CL and 45.7% (range: 22.2%–105.4%; n=7) for V. Residual unexplained variability (RUV) was predominantly modeled using proportional error models, demonstrating a range of 4.15–34% (n=4). Notably, Zhang et al20 reported significantly higher BSV for CL (65.9%) in pediatric populations compared to other studies.
Internal evaluations were conducted for all pharmacokinetic (PK) models. Goodness-of-fit (GOF) plots, demonstrating predicted versus observed value correlations, were the most frequently employed validation method. All studies17–24 incorporated model-based simulations to characterize the PK profiles of TEC. Furthermore, six studies17–20,23,24 extended simulations to quantify covariate effects and derive optimized dosing regimens.
The simulations in the included studies were based on two PK/PD targets: AUC24/MIC and Cmin. PTA simulations focused solely on MRSA, with each PPK model evaluated using strain-specific MIC data (Figure 6). This study found that MIC and Cmin values significantly affected drug efficacy in different pediatric populations. When the MIC was 2 mg/L, the PTA of all populations did not exceed 90%, indicating that existing standard treatment regimens were ineffective and medication strategies needed adjustment. When the Cmin was 10 mg/L, there were differences in attainment rates among populations. The attainment rates for preterm infants, neonates, infants, children, and adolescents were 100% (with a sample size of 1 study), 50% (from 2 studies), 42.86% (from 7 studies), 28.57% (from 7 studies), and 33.33% (from 3 studies), respectively. The PTA results suggested that for pediatric patients, drug dosages should be determined by combining the target Cmin and MIC values.
 |
Figure 6 The probability target achievement of TEC in included studies. The gray dashed lines represent a 90% target attainment rate, while the green vertically oriented dashed lines correspond to the respective clinical breakpoints for MIC specific to MRSA and Cmin of TEC.
|
Teicoplanin, a glycopeptide antibiotic, is widely used for methicillin-resistant Staphylococcus aureus (MRSA) infections. Although numerous PK studies on TEC existed, few had systematically evaluated the physiological and pathological factors contributing to its exposure variability. To our knowledge, this study represented the first systematic evaluation of PPK models for TEC, specifically addressing the need for dose adjustment in pediatric populations.
The PK of TEC exhibited significant differences among pediatric patients at different age stages. The median CL values for children were similar to those of preterm infants and infants. However, the median CL in neonates was significantly higher than in adolescents. The difference in CL between neonates and adolescents could be explained by several key factors. Studies28–30 had shown that neonates exhibited nearly twice the glomerular filtration rate per unit body weight compared to adolescents. This was accompanied by significantly increased expression of renal tubular transporters, including organic anion transporters (OATs) and organic anion transporting polypeptides (OATPs), which markedly enhanced drug excretion. Moreover, neonates have only about 50% of the α1-acid glycoprotein (AAG) levels observed in adolescents. This lower AAG concentration leads to higher free drug fractions, which are more readily cleared by the kidneys.31–33 Of equal importance, neonates exhibit a larger volume of distribution and higher extracellular fluid ratio, which enhances initial drug clearance. In contrast, adolescents show lower clearance as metabolic enzymes mature toward hepatic dominance.34–36
The elimination of TEC was influenced by the maturity of renal function and body composition. Studies23,36 show that CL increased significantly with PMA growth. In neonates (PMA: 23.7–42.4 weeks), the CL increased 4.7-fold, rising from 0.00473 to 0.0223 L/h. In infants (PMA: 30.6–136.4 weeks), the CL escalated 5.4-fold, from 0.0299 to 0.161 L/h. While in children (PMA: 134–878 weeks), it stabilized at a level ranging from 0.520 to 0.536 L/h. This PMA-related pattern correlated with glomerular filtration rate and muscle mass development.37,38 Thus, critically ill neonates with lower PMA require dosage adjustment to avoid accumulation, while infants nearing 1 year may need higher weight-normalized doses to maintain therapeutic levels.
The study by Yamada et al23 demonstrated that PNA significantly affected the CL of TEC in pediatrics with PMA < 48 weeks. During the first 27 days of life, the CL of TEC remained at a low level of 0.0126–0.0141 L/h, only 1.3–1.4% of the reference value (0.990 L/h), confirming immature renal elimination pathways in the first month of life. In contrast, during infancy (36.5–657 days), CL increased markedly to 0.134 L/h, representing 13.5% of the values. This 10.6-fold rise from neonatal levels reflected rapid renal maturation in this developmental stage. These results indicated that PNA was a critical determinant of TEC dosing, particularly during the transition from neonates to infants, where clearance capacity undergoes a dramatic surge.21 Consequently, dose adjustments were essential for neonates due to their substantially lower clearance capacity. The nonlinear relationship between CL and PNA necessitates age-stratified dosing regimens for this special population.
The CL of TEC was influenced by ALB levels, particularly in neonates and infants.39 Yamada et al23 demonstrated that higher ALB values were associated with reduced the CL of TEC in these populations. In neonates with ALB levels ranging from 1.1 to 5.5 g/dL, the CL decreased from 0.0370 to 0.00650 L/h, representing an approximately 5.7-fold reduction. Similarly, in infants (ALB: 2–5.1 g/dL), the CL declined from 0.245 to 0.0892 L/h, with a 2.8-fold difference. This inverse correlation suggested that albumin binding may limit the free fraction of TEC, thereby reducing its clearance.39 Consequently, dose adjustment may be required for neonates and infants with elevated ALB levels to ensure the attainment of effective therapeutic concentrations.
The CL of TEC was significantly influenced by renal function parameters. In pediatric populations, both eGFR and CLcr had been independently incorporated into PPK models as key covariates affecting TEC elimination. Studies18,19,21,23 demonstrated that the developmental stage of renal function substantially impacted TEC clearance. Results19,23 indicated that variations in eGFR could lead to a 6.3-fold fluctuation in TEC clearance, ranging from 0.151 to 0.949 times the reference value. In comparison, CLcr variations exerted a more pronounced effect on clearance, showing up to a 7.1-fold difference, from 98.8% to 703.9% of the reference value.18,21 This discrepancy mainly arose because CLcr more directly reflected glomerular filtration function and was independent of muscle metabolism interference, thus showing greater sensitivity to changes in renal function.40
Multiple studies20,23 had demonstrated a significant inverse correlation between Scr levels and TEC clearance. Specifically, when neonatal Scr concentrations increased from 17.68 to 159.12 μmol/L, TEC clearance decreased from 0.0250 to 0.00792 L/h, representing a 3.1-fold reduction. Notably, in infant populations, as Scr rose from 8.84 to 150.3 μmol/L, TEC clearance showed a more pronounced decline from 0.357 to 0.0491 L/h, reflecting a 7.3-fold difference. This Scr-dependent clearance pattern was particularly pronounced in early infancy, showing a close correlation with the rapid developmental changes in renal function that were characteristic of this period.41
One study24 reported that during continuous kidney replacement therapy (CKRT), the 30% reduction in TEC clearance was mainly attributed to the drug being adsorbed onto the dialysis membrane and the convective loss of the unbound fraction during ultrafiltration.42 Notably, the study further revealed that the surface area of the filter had a significant impact on the CL of TEC in patients undergoing CKRT. Medium and large filters could notably enhance the CL of TEC by 2.58-fold and 4.04-fold, respectively. This effect was likely attributed to their larger surface areas, which facilitated more efficient drug clearance.43
In this study, the notable influence of MIC and Cmin on drug efficacy in various pediatric groups stemmed from multiple factors. Literature indicated that preterm infants and neonates had immature metabolism and clearance mechanisms, which resulted in unique PK profiles.44 Their reduced drug-processing ability leads to diverse drug concentrations, affecting the achievement of PK/PD targets like Cmin. When Cmin was set at 10 mg/L, the significant variation in probability of target attainment (PTA) across pediatric age groups revealed the key role of age-related physiology.45 Preterm infants, with less-developed drug-elimination pathways, more easily reached the Cmin target in the single included study. In contrast, older children and adolescents have more active metabolisms, leading to lower attainment rates. When MIC was 2 mg/L, all age groups failed to achieve a PTA above 90%, which suggested that current standard treatments may be inadequate. The reduced efficacy across all pediatric groups likely stemmed from elevated MRSA resistance (MIC = 2 mg/L) to TEC, which compromised its growth-inhibitory capacity.46
In summary, these results highlighted the need for personalized dosing in pediatric patients. Considering both target Cmin and AUC24/MIC, healthcare providers could optimize drug treatment, factoring in the distinct physiology and resistance patterns of different pediatric age groups.
The establishment of the 90% PTA threshold as a standard is based on several key considerations. First, populations such as children and patients with renal impairment exhibit significant pharmacokinetic variability. Monte Carlo simulations quantify this variability to assess the robustness of dosing regimens, with the >90% threshold ensuring coverage of the vast majority of patients and reducing treatment failures caused by individual differences. Second, although therapeutic drug monitoring (TDM) is the gold standard, its immediate implementation in medical institutions remains limited. A dosing regimen validated by Monte Carlo simulations with >90% PTA can provide the optimal choice for initial treatment before TDM results are available; if PTA is below 90%, it indicates the need for dosage adjustment or early initiation of TDM. Finally, relevant studies have also set PTA at ≥90%, demonstrating that this standard ensures most pediatric patients achieve drug exposures associated with effective bacterial suppression, thereby indirectly reducing the risk of drug resistance.47,48
It is important to note that despite the consistent core findings across most included studies, certain conclusions regarding TEC pharmacokinetic covariates remain inconsistent. For instance, discrepancies exist regarding the impact of ALB on TEC clearance: Yamada et al23 reported a 5.7-fold reduction in CL with increasing ALB in neonates, whereas Aulin et al22 did not identify ALB as a significant covariate—potentially attributed to differences in study populations (neonates vs broader pediatric cohorts) and ALB measurement ranges (1.1–5.5 g/dL vs 2–5.1 g/dL). Similarly, variations in the effect of PNA on CL were noted between Kontou et al21 (no significant association) and Yamada et al23 (10.6-fold CL increase in infancy vs neonates), likely driven by differences in PNA stratification (6–17.7 days vs 1–657 days) and concurrent adjustment for PMA in the latter.
To date, PK/PD studies on TEC remain limited, particularly those focusing on pediatric patients. A critical gap exists in clarifying the quantitative relationship between pharmacodynamic indicators and adverse effects. Therefore, larger prospective PK/PD studies in pediatric populations are essential to better characterize the associations among dose, drug exposure, and therapeutic response.
This review is subject to a number of limitations. First, we only included English-language literature, which may have led to the omission of potentially valuable studies from non-English-speaking regions. Second, we did not incorporate nonparametric PPK models, a choice that may result in an incomplete interpretation of certain analytical approaches. Third, the absence of external validation using an independent dataset, which may limit the generalizability of the models to other clinical settings. Notably, plasma exposure is a surrogate marker for the infection site. This discrepancy may cause deviations between the predicted PTA and actual clinical outcomes. Treatment failure cannot be assumed based solely on PTA targets, which are derived from combining drug concentrations in the central compartment and microbiological MICs over time.
This systematic review of published PPK studies on TEC highlights significant PK variability of TEC across pediatric populations, with neonates exhibiting a higher weight-normalized CL than adolescents. Methodologically, this review incorporates a distinct innovative approach through the integrated application of VPD and Monte Carlo Simulations (MCS). This combined framework enables systematic comparison of PK parameters across pediatric age cohorts and rigorous evaluation of the PTA, thereby furnishing a robust technical underpinning for the key findings presented herein. The key covariates influencing TEC pharmacokinetics include body weight, PMA, renal function parameters (eGFR, CLcr, Scr), ALB levels, and CKRT status. Furthermore, dosage regimen adjustments should be considered based on pediatric age subgroups and pathogen-specific MICs, particularly for infections caused by MRSA with an MIC of 2 mg/L. Notably, this analysis establishes a practical framework for optimizing future clinical dosing regimens of TEC. For example, preterm infants may require lower maintenance doses to prevent excessive drug exposure, whereas neonates and children may need higher weight-normalized doses to achieve therapeutic targets. Additionally, further large-scale prospective population studies are essential to clarify the dose-exposure-response relationship of TEC in pediatric populations. Nevertheless, significant external validation will be required before clinical implementation.
The authors would like to thank Xiping Li from Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan for his critical comments and suggestions regarding the data visualization.
This research was financially supported by the Foundation of Health Commission of Anhui Province (Grant No. AHWJ2023BAa20159).
The authors declare no conflicts of interest in this work.
1. Behring AH, Mariani C, Gerbi M, et al. Appropriateness of loading dose and TDM in patients treated with teicoplanin: a single-centre observational retrospective cohort study comparing single-daily and thrice-weekly regimens. J Antimicrob Chemother. 2025;80(6):1535–1542. doi:10.1093/jac/dkaf097
2. Beltrametti F, Consolandi A, Carrano L, et al. Resistance to glycopeptide antibiotics in the teicoplanin producer is mediated by van gene homologue expression directing the synthesis of a modified cell wall peptidoglycan. Antimicrob Agents Chemother. 2007;51(4):1135–1141. doi:10.1128/aac.01071-06
3. David MZ, Daum RS. Treatment of staphylococcus aureus infections. Curr Top Microbiol Immunol. 2017;2017:325–383.
4. Lode H, Boeckh M, Höffken G, et al. Comparative pharmacokinetics of glycopeptide antibiotics, and the influence of teicoplanin on granulocyte function. Scand J Infect Dis Suppl. 1990;72:9–13.
5. Mouton JWA, De Clercq A, De Paepe P, et al. Pharmacokinetics and target attainment of teicoplanin: a systematic review. Clin Pharmacokinet. 2025;64:467–509. doi:10.1007/s40262-025-01483-7
6. Matsumoto K, Watanabe E, Kanazawa N, et al. Pharmacokinetic/pharmacodynamic analysis of teicoplanin in patients with MRSA infections. Clin Pharmacol. 2016;8:15–18. doi:10.2147/cpaa.S96143
7. Sato Y, Hiramatsu K, Suzuki Y, et al. Optimal trough concentration of teicoplanin in febrile neutropenic patients with hematological malignancy. Chemotherapy. 2018;63(1):29–34. doi:10.1159/000481725
8. Hanai Y, Takahashi Y, Niwa T, et al. Optimal trough concentration of teicoplanin for the treatment of methicillin-resistant Staphylococcus aureus infection: a systematic review and meta-analysis. J Clin Pharm Ther. 2021;46(3):622–632. doi:10.1111/jcpt.13366
9. Hanai Y, Takahashi Y, Niwa T, et al. Clinical practice guidelines for therapeutic drug monitoring of teicoplanin: a consensus review by the Japanese society of chemotherapy and the Japanese society of therapeutic drug monitoring. J Antimicrob Chemother. 2022;77(4):869–879. doi:10.1093/jac/dkab499
10. Tsuji Y. Hospital pharmacometrics for optimal individual administration of antimicrobial agents for anti-methicillin-resistant staphylococcus aureus infected patients. Biol Pharm Bull. 2021;44(9):1174–1183. doi:10.1248/bpb.b21-00002
11. Merino-Bohórquez V, Docobo-Pérez F, Sojo J, et al. Population pharmacokinetics and pharmacodynamics of fosfomycin in non-critically ill patients with bacteremic urinary infection caused by multidrug-resistant Escherichia coli. Clin Microbiol Infect. 2018;24(11):1177–1183. doi:10.1016/j.cmi.2018.02.005
12. Chen CY, Xie M, Gong J, et al. Population pharmacokinetic analysis and dosing regimen optimization of teicoplanin in critically ill patients with sepsis. Front Pharmacol. 2023;14:1132367. doi:10.3389/fphar.2023.1132367
13. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:
14. Kanji S, Hayes M, Ling A, et al. Reporting guidelines for clinical pharmacokinetic studies: the ClinPK statement. Clin Pharmacokinet. 2015;54(7):783–795. doi:10.1007/s40262-015-0236-8
15. Jamsen KM, McLeay SC, Barras MA, Green B. Reporting a population pharmacokinetic-pharmacodynamic study: a journal’s perspective. Clin Pharmacokinet. 2014;53(2):111–122. doi:10.1007/s40262-013-0114-1
16. Duffull SB, Wright DF. What do we learn from repeated population analyses? Br J Clin Pharmacol. 2015;79(1):40–47. doi:10.1111/bcp.12233
17. Lukas JC, Karikas G, Gazouli M, Kalabalikis P, Hatzis T, Macheras P. Pharmacokinetics of teicoplanin in an ICU population of children and infants. Pharm Res. 2004;21(11):2064–2071. doi:10.1023/b:pham.0000048198.56873.d8
18. Zhao W, Zhang D, Storme T, Baruchel A, Declèves X, Jacqz-Aigrain E. Population pharmacokinetics and dosing optimization of teicoplanin in children with malignant haematological disease. Br J Clin Pharmacol. 2015;80(5):1197–1207. doi:10.1111/bcp.12710
19. Gao L, Xu H, Ye Q, et al. Population pharmacokinetics and dosage optimization of teicoplanin in children with different renal Functions. Front Pharmacol. 2020;11:552. doi:10.3389/fphar.2020.00552
20. Zhang T, Sun D, Shu Z, et al. Population pharmacokinetics and model-based dosing optimization of teicoplanin in pediatric patients. Front Pharmacol. 2020;11:594562. doi:10.3389/fphar.2020.594562
21. Kontou A, Sarafidis K, Begou O, et al. Population pharmacokinetics of teicoplanin in preterm and term neonates: is it time for a new dosing regimen? Antimicrob Agents Chemother. 2020;64(4). doi:10.1128/aac.01971-19
22. Aulin LBS, De Paepe P, Dhont E, et al. Population Pharmacokinetics of unbound and total teicoplanin in critically Ill pediatric patients. Clin Pharmacokinet. 2021;60(3):353–363. doi:10.1007/s40262-020-00945-4
23. Yamada T, Emoto C, Fukuda T, et al. Optimal teicoplanin dosing regimen in neonates and children developed by leveraging real-world clinical information. Ther Drug Monit. 2022;44(3):404–413. doi:10.1097/ftd.0000000000000930
24. Butragueño-Laiseca L, García-Orueta G, Riva N, et al. Population pharmacokinetic analysis of teicoplanin in paediatric patients, including those receiving continuous kidney replacement therapy: a prospective cohort study. J Antimicrob Chemother. 2025;80(3):868–875. doi:10.1093/jac/dkaf012
25. Christians U, Klawitter J, Clavijo CF. Bioequivalence testing of immunosuppressants: concepts and misconceptions. Kidney Int Suppl. 2010;(115):S1–7. doi:10.1038/ki.2009.504
26. Pea F, Brollo L, Viale P, Pavan F, Furlanut M. Teicoplanin therapeutic drug monitoring in critically ill patients: a retrospective study emphasizing the importance of a loading dose. J Antimicrob Chemother. 2003;51(4):971–975. doi:10.1093/jac/dkg147
27. Chang HJ, Hsu PC, Yang CC, et al. Influence of teicoplanin MICs on treatment outcomes among patients with teicoplanin-treated methicillin-resistant staphylococcus aureus bacteraemia: a hospital-based retrospective study. J Antimicrob Chemother. 2012;67(3):736–741. doi:10.1093/jac/dkr531
28. Staub E, Bolisetty S, Allegaert K, Raaijmakers A. neonatal kidney function, injury and drug dosing: a contemporary review. Children. 2025;12(3). doi:10.3390/children12030339
29. Smeets NJL, IntHout J, van der Burgh MJP, Schwartz GJ, Schreuder MF, de Wildt SN. Maturation of GFR in term-born neonates: an individual participant data meta-analysis. J Am Soc Nephrol. 2022;33(7):1277–1292. doi:10.1681/asn.2021101326
30. Wu Y, Allegaert K, Flint RB, et al. Prediction of glomerular filtration rate maturation across preterm and term neonates and young infants using inulin as marker. Aaps j. 2022;24(2):38. doi:10.1208/s12248-022-00688-z
31. Thompson LE, Joy MS. Endogenous markers of kidney function and renal drug clearance processes of filtration, secretion, and reabsorption. Curr Opin Toxicol. 2022;31. doi:10.1016/j.cotox.2022.03.005
32. Ligi I, Boubred F, Grandvuillemin I, Simeoni U. The neonatal kidney: implications for drug metabolism and elimination. Curr Drug Metab. 2013;14(2):174–177.
33. Lu H, Rosenbaum S. Developmental pharmacokinetics in pediatric populations. J Pediatr Pharmacol Ther. 2014;19(4):262–276. doi:10.5863/1551-6776-19.4.262
34. Mahmood I. Prediction of total and renal clearance of renally secreted drugs in neonates and infants (≤3 months of age). J Clin Trans Res. 2022;8(6):445–452.
35. Alcorn J, McNamara PJ. Ontogeny of hepatic and renal systemic clearance pathways in infants: part I. Clin Pharmacokinet. 2002;41(12):959–998. doi:10.2165/00003088-200241120-00003
36. Rhodin MM, Anderson BJ, Peters AM, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24(1):67–76. doi:10.1007/s00467-008-0997-5
37. Mahmood I, Staschen CM. Prediction of human glomerular filtration rate from preterm neonates to adults: evaluation of predictive performance of several empirical models. Aaps j. 2016;18(2):445–454. doi:10.1208/s12248-016-9868-3
38. Groothof D, Post A, Polinder-Bos HA, et al. Muscle mass and estimates of renal function: a longitudinal cohort study. J Cachexia Sarcopenia Muscle. 2022;13(4):2031–2043. doi:10.1002/jcsm.12969
39. Yano R, Nakamura T, Tsukamoto H, et al. Variability in teicoplanin protein binding and its prediction using serum albumin concentrations. Ther Drug Monit. 2007;29(4):399–403. doi:10.1097/FTD.0b013e3180690755
40. Khader NA, Kamath VG, Kamath SU, Rao IR, Prabhu AR. Kidney function estimation equations: a narrative review. Ir J Med Sci. 2025;194(2):725–743. doi:10.1007/s11845-025-03874-y
41. Starr MC, Griffin RL, Harer MW, et al. Acute kidney injury defined by fluid-corrected creatinine in premature neonates: a secondary analysis of the PENUT randomized clinical trial. JAMA Network Open. 2023;6(8):e2328182. doi:10.1001/jamanetworkopen.2023.28182
42. Yoshimoto K, Matsuura R, Komaru Y, et al. Solute clearance evaluation and filter clotting prediction in continuous renal replacement therapy. J Clin Med. 2023;12(24). doi:10.3390/jcm12247703
43. Tsujimoto Y, Fujii T. How to prolong filter life during continuous renal replacement therapy? Crit Care. 2022;26(1):62. doi:10.1186/s13054-022-03910-8
44. Bardol M, Norman E, Lagercrantz H, Fellman V, Standing JF. Fentanyl dosage for preterm infants suggested by a pharmacokinetic, -dynamic, and -genetic model. Pediatr Res. 2025;97(1):239–245. doi:10.1038/s41390-024-03404-z
45. Strenger V, Hofer N, Rödl S, et al. Age- and gender-related differences in teicoplanin levels in paediatric patients. J Antimicrob Chemother. 2013;68(10):2318–2323. doi:10.1093/jac/dkt176
46. Mandell JB, Gish C, Cappellini AJ, et al. Methicillin resistant staphylococcus aureus mazEF expression promotes infections by influencing cellular growth, antibiotic sensitivity, and formation of biofilms. Sci Rep. 2024;14(1):21269. doi:10.1038/s41598-024-70829-1
47. Li XP, Liu XQ, Mao JH, Liu D, Jiao Z. Evaluation of population pharmacokinetic models of micafungin: implications for dosing regimen optimization in critically Ill patients. Pharmaceutics. 2024;16(9):1145. doi:10.3390/pharmaceutics16091145
48. Zhou YZ, Huang C, Ji JR, et al. Optimization of dosing regimens of vancomycin and teicoplanin in treatment of methicillin-resistant staphylococcal bloodstream infection based on monte carlo simulation. Herald Med. 2022;41(7):1026–1032. doi:10.3870/j.issn.1004-0781.2022.07.017

Indian National Film Award-winning actor Parvathy Thiruvothu is set to spearhead Don Palathara‘s next directorial venture, co-starring the acclaimed filmmaker-actor Dileesh Pothan.
Thiruvothu, whose performance in “Take Off” (2017)…