In the latest episode of Chinese-Exclusive Smartphone Launch: Season 10/25, Redmi (or Xiaomi) unsurprisingly launches their latest and greatest flagship killer, the K90 Pro Max.
First “Pro Max” Midranger
Much like the its…

In the latest episode of Chinese-Exclusive Smartphone Launch: Season 10/25, Redmi (or Xiaomi) unsurprisingly launches their latest and greatest flagship killer, the K90 Pro Max.
Much like the its…

Ad hoc announcement pursuant to Art. 53 LR
1. Constant currencies (cc), core results and free cash flow are non-IFRS measures. An explanation of non-IFRS measures can be found on page 42 of the Condensed Interim Financial Report. Unless otherwise noted, all growth rates in this Release refer to same period in prior year. 2. Please see detailed guidance assumptions on page 7.
Basel, October 28, 2025 – Commenting on Q3 2025 results, Vas Narasimhan, CEO of Novartis, said:
“Novartis delivered solid financial performance in Q3, more than offsetting the impact of increasing generic erosion in the US. Our key growth drivers performed well, including Kisqali, Kesimpta, Pluvicto and Scemblix. Importantly, we achieved FDA approval for Rhapsido in CSU and positive Phase III readouts for ianalumab in Sjogren’s disease – two assets with pipeline-in-a-pill potential that could underpin our growth through 2030 and beyond. In addition, we completed several deals in the quarter to further strengthen our pipeline in core therapeutic areas. We remain well on track to achieve our guidance for 2025 and over the mid-term.”
| Key figures | |||||||||
| Q3 2025 | Q3 2024 | % change | 9M 2025 | 9M 2024 | % change | ||||
| USD m | USD m | USD | cc | USD m | USD m | USD | cc | ||
| Net sales | 13 909 | 12 823 | 8 | 7 | 41 196 | 37 164 | 11 | 11 | |
| Operating income | 4 501 | 3 627 | 24 | 27 | 14 028 | 11 014 | 27 | 31 | |
| Net income | 3 930 | 3 185 | 23 | 25 | 11 563 | 9 119 | 27 | 29 | |
| EPS (USD) | 2.04 | 1.58 | 29 | 31 | 5.94 | 4.50 | 32 | 35 | |
| Free cash flow | 6 217 | 5 965 | 4 | 15 941 | 12 618 | 26 | |||
| Core operating income | 5 460 | 5 145 | 6 | 7 | 16 960 | 14 635 | 16 | 18 | |
| Core net income | 4 330 | 4 133 | 5 | 6 | 13 522 | 11 822 | 14 | 17 | |
| Core EPS (USD) | 2.25 | 2.06 | 9 | 10 | 6.94 | 5.83 | 19 | 21 | |
Strategy
Our focus
Novartis is a “pure-play” innovative medicines company. We have a clear focus on four core therapeutic areas (cardiovascular-renal-metabolic, immunology, neuroscience and oncology), with multiple significant in-market and pipeline assets in each of these areas, that address high disease burden and have substantial growth potential. In addition to two established technology platforms (chemistry and biotherapeutics), three emerging platforms (gene & cell therapy, radioligand therapy and xRNA) are being prioritized for continued investment into new R&D capabilities and manufacturing scale. Geographically, we are focused on growing in our priority geographies – the US, China, Germany and Japan.
Our priorities
Financials
Third quarter
Net sales were USD 13.9 billion (+8%, +7% cc), with volume contributing 16 percentage points to growth. Generic competition had a negative impact of 7 percentage points, driven by Promacta, Tasigna and Entresto generics in the US. Pricing had a negative impact of 2 percentage points, driven by revenue deduction adjustments mainly in the US. Currency had a positive impact of 1 percentage point.
Operating income was USD 4.5 billion (+24%, +27% cc), mainly driven by higher net sales and lower impairments, partly offset by higher R&D investments.
Net income was USD 3.9 billion (+23%, +25% cc), mainly driven by higher operating income. EPS was USD 2.04 (+29%, +31% cc), benefiting from the lower weighted average number of shares outstanding.
Core operating income was USD 5.5 billion (+6%, +7% cc), mainly driven by higher net sales, partly offset by higher R&D investments. Core operating income margin was 39.3% of net sales (-0.8 percentage points, stable in cc).
Core net income was USD 4.3 billion (+5%, +6% cc), mainly due to higher core operating income, partly offset by other core financial income and expense. Core EPS was USD 2.25 (+9%, +10% cc), benefiting from the lower weighted average number of shares outstanding.
Free cash flow amounted to USD 6.2 billion (+4% USD), compared with USD 6.0 billion in the prior-year quarter, driven by higher net cash flows from operating activities.
Nine months
Net sales were USD 41.2 billion (+11%, +11% cc), with volume contributing 14 percentage points to growth. Generic competition had a negative impact of 3 percentage points, while pricing and currency had no impact.
Operating income was USD 14.0 billion (+27%, +31% cc), mainly driven by higher net sales and lower impairments, partly offset by higher investments behind priority brands and launches.
Net income was USD 11.6 billion (+27%, +29% cc), mainly driven by higher operating income. EPS was USD 5.94 (+32%, +35% cc), benefiting from the lower weighted average number of shares outstanding.
Core operating income was USD 17.0 billion (+16%, +18% cc), mainly driven by higher net sales, partly offset by higher investments behind priority brands and launches. Core operating income margin was 41.2% of net sales, increasing 1.8 percentage points (2.5 percentage points cc).
Core net income was USD 13.5 billion (+14%, +17% cc), mainly due to higher core operating income. Core EPS was USD 6.94 (+19%, +21% cc), benefiting from the lower weighted average number of shares outstanding.
Free cash flow amounted to USD 15.9 billion (+26% USD), compared with USD 12.6 billion in the prior-year period, driven by higher net cash flows from operating activities.
Q3 priority brands
Underpinning our financial results in the quarter is a continued focus on key growth drivers (ranked in order of contribution to Q3 growth) including:
| Kisqali | (USD 1 329 million, +68% cc) sales grew strongly across all regions, including +91% growth in the US with strong momentum from the recently launched early breast cancer indication as well as continued share gains in metastatic breast cancer. |
| Kesimpta | (USD 1 222 million, +44% cc) sales grew across all regions driven by increased demand and strong access. |
| Pluvicto | (USD 564 million, +45% cc) showed sustained demand growth in the US following the pre-taxane metastatic castration-resistant prostate cancer (mCRPC) approval, as well as continued access expansion ex-US in the post-taxane mCRPC setting, with 25 countries now approved including Japan. |
| Scemblix | (USD 358 million, +95% cc) sales grew across all regions, demonstrating the continued high unmet need in CML, with strong momentum from the early-line indication in the US and Japan. |
| Leqvio | (USD 308 million, +54% cc) continued steady growth across all regions, with a focus on increasing account and patient adoption, and continuing medical education. |
| Fabhalta | (USD 149 million, +236% cc) sales grew, reflecting market share gains in PNH globally and continued launch progress in IgAN and C3G in the US. |
| Lutathera | (USD 213 million, +11% cc) sales grew mainly in the US, Japan and Europe due to increased demand and earlier-line adoption. |
| Cosentyx | (USD 1 698 million, -1% cc) sales were broadly stable, as strong volume growth in the US was partially offset by higher revenue deductions, and ex-US declined due to a one-time price effect in the prior year. Novartis remains confident in Cosentyx USD 8 billion+ peak sales guidance. |
| Zolgensma | (USD 301 million, -5% cc) sales declined reflecting a lower incidence of SMA compared to prior year. |
Net sales of the top 20 brands in the third quarter and nine months
| Q3 2025 | % change | 9M 2025 | % change | |||
| USD m | USD | cc | USD m | USD | cc | |
| Entresto | 1 877 | 1 | -1 | 6 495 | 15 | 15 |
| Cosentyx – excl. revenue deduction adjust.* |
1 698 | 0 5 |
-1 4 |
4 861 | 7 9 |
7 9 |
| Kisqali | 1 329 | 69 | 68 | 3 462 | 62 | 63 |
| Kesimpta | 1 222 | 46 | 44 | 3 198 | 41 | 40 |
| Tafinlar + Mekinist | 550 | 3 | 1 | 1 675 | 9 | 9 |
| Jakavi | 539 | 8 | 4 | 1 555 | 7 | 6 |
| Promacta/Revolade | 362 | -36 | -38 | 1 410 | -14 | -14 |
| Pluvicto | 564 | 46 | 45 | 1 389 | 33 | 33 |
| Ilaris | 473 | 27 | 26 | 1 369 | 25 | 24 |
| Xolair | 440 | 5 | 3 | 1 339 | 8 | 8 |
| Tasigna | 221 | -47 | -48 | 925 | -27 | -26 |
| Zolgensma | 301 | -2 | -5 | 925 | -3 | -4 |
| SandostatinGroup | 302 | -1 | -1 | 922 | -5 | -5 |
| Scemblix | 358 | 97 | 95 | 894 | 85 | 84 |
| Leqvio | 308 | 56 | 54 | 863 | 63 | 61 |
| Lutathera | 213 | 12 | 11 | 613 | 15 | 14 |
| ExforgeGroup | 176 | 1 | 0 | 546 | 0 | 2 |
| Lucentis | 148 | -40 | -42 | 510 | -39 | -39 |
| DiovanGroup | 143 | -5 | -5 | 447 | -1 | 0 |
| GalvusGroup | 126 | -21 | -20 | 373 | -19 | -16 |
| Top 20 brands total | 11 350 | 10 | 9 | 33 771 | 14 | 14 |
*Sales growth impacted by a one-time revenue deduction adjustment in the US
R&D update – key developments from the third quarter
New approvals
| Rhapsido (remibrutinib) |
Rhapsido was approved by the FDA as an oral treatment for adult patients with chronic spontaneous urticaria (CSU) who remain symptomatic despite H1 antihistamine treatment. It is the first FDA-approved Bruton’s tyrosine kinase inhibitor (BTKi) for CSU. Remibrutinib is also in Phase III development for chronic inducible urticaria, hidradenitis suppurativa and food allergy, as well as multiple sclerosis and myasthenia gravis. |
Regulatory updates
| Scemblix (asciminib) | The CHMP of the EMA adopted a positive opinion and recommended granting marketing authorization for Scemblix for the treatment of adult patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase (Ph+ CML-CP) in all lines of treatment. |
Results from ongoing trials and other highlights
| Ianalumab (VAY736) |
The Phase III NEPTUNUS-1 and -2 trials evaluating ianalumab in adults with active Sjögren’s disease met their primary endpoint, showing statistically significant improvements in disease activity as measured by a reduction in ESSDAI compared to placebo. Ianalumab was well tolerated and demonstrated a favorable safety profile, supporting its potential to become the first targeted treatment for this chronic autoimmune disease. Novartis plans to submit ianalumab to health authorities globally and was granted Fast Track Designation by the FDA.
In the Phase III VAYHIT2 trial, ianalumab plus eltrombopag significantly extended the time to treatment failure compared to placebo plus eltrombopag in adult patients with primary immune thrombocytopenia (ITP), previously treated with corticosteroids. The safety profile was consistent with previous studies. Data will be presented at an upcoming medical meeting and included in regulatory submissions in 2027. Ianalumab is also in Phase III development for systemic lupus erythematosus, lupus nephritis and warm autoimmune hemolytic anemia. |
| Pluvicto (lutetium Lu177 vipivotide tetraxetan) |
In the Phase III PSMAddition trial, Pluvicto plus standard-of-care (SoC) reduced risk of progression or death by 28% versus SoC alone, with a positive trend in overall survival in patients with PSMA+ metastatic hormone-sensitive prostate cancer (mHSPC). Safety remained consistent with PSMAfore and VISION trials. Data presented at ESMO. |
| Kisqali (ribociclib) |
The five-year analysis of the pivotal Phase III NATALEE trial in the broadest population of high-risk stage II and III HR+/HER2- early breast cancer (eBC) showed the addition of Kisqali to endocrine therapy (ET) reduced the risk of recurrence by 28.4% compared to ET alone. Data also showed a 29.1% risk reduction in distant disease-free survival, a positive trend in overall survival, and no new safety signals. Data presented at ESMO. |
| Cosentyx (secukinumab) |
The Phase III REPLENISH study met its primary endpoint, with Cosentyx demonstrating statistically significant and clinically meaningful sustained remission compared to placebo at week 52 in adults with relapsing polymyalgia rheumatica (PMR). Full data will be presented at an upcoming medical congress and submitted to health authorities in 2026. |
| Fabhalta (iptacopan) |
In the Phase III APPLAUSE-IgAN final analysis, Fabhalta demonstrated statistically significant, clinically meaningful superiority compared to placebo in slowing IgAN progression measured by annualized total slope of estimated glomerular filtration rate (eGFR) decline over two years. Full data will be presented at future medical meetings and included in regulatory submissions in 2026. |
| Leqvio (Inclisiran) |
In the Phase IV V-DIFFERENCE study, 85% of patients with hypercholesterolemia who had not reached guideline-recommended LDL-C targets despite optimized lipid-lowering therapy (LLT) achieved their goals with Leqvio plus LLT, versus 31% with placebo plus LLT, with benefits evident in as early as 30 days. Leqvio also reduced LDL-C by 59% over 360 days, outperforming placebo plus LLT by 35%. Data presented at ESC. |
| Entresto (sacubitril/ valsartan) |
Data from the Phase IV PARACHUTE-HF study in patients with heart failure with reduced ejection fraction due to chronic Chagas disease showed that Entresto outperformed enalapril on a composite endpoint of cardiovascular death, heart failure hospitalization or NT-proBNP change. Entresto was well tolerated, with no new safety signals identified. Data presented at ESC. |
| Kesimpta (ofatumumab) |
In the ARTIOS Phase IIIb study, patients with RMS who switched to Kesimpta after breakthrough disease on fingolimod or fumarate-based therapies showed a substantial reduction in disease activity. This was reflected in a low annualized relapse rate (ARR of 0.06 over 96 weeks), near-complete suppression of MRI activity, and over 90% of participants achieving no evidence of disease activity (NEDA-3). No new safety concerns were identified, regardless of prior disease-modifying treatment.
In the separate ALITHIOS open-label extension study, more than 90% of naïve patients receiving Kesimpta showed no evidence of disease activity (NEDA-3) at 7 years, with no new safety concerns, reinforcing the benefit of introducing Kesimpta early. Data from both studies presented at ECTRIMS. |
| Selected transactions | Novartis entered into an agreement to acquire Tourmaline Bio, a clinical-stage biopharmaceutical company developing pacibekitug, a Phase III-ready anti-IL-6 monoclonal antibody for atherosclerotic cardiovascular disease (ASCVD). In Phase II, pacibekitug reduced median high-sensitivity C-reactive protein (hsCRP) levels by up to 86% compared to placebo, with similar incidence rates of adverse events and serious adverse events. The transaction is expected to close on October 28, 2025.
Novartis entered a second collaboration with Monte Rosa Therapeutics, in addition to the existing license agreement for VAV1 degraders, announced in October 2024. Under the new agreement, Novartis receives an exclusive license to an undisclosed discovery target and options to license two programs from Monte Rosa’s preclinical immunology portfolio. Novartis continued its collaboration with Argo Biopharma, adding two new agreements: an exclusive license to an siRNA candidate currently in IND-enabling studies and expected to enter Phase I in 2026, and an option to exclusively license two second-generation siRNA molecules currently in development, with a right of first negotiation to the Phase II ANGPTL3 program. Novartis entered into a global licensing and collaboration agreement with Arrowhead Pharmaceuticals for ARO-SNCA, a preclinical-stage siRNA therapy targeting alpha-synuclein for the treatment of synucleinopathies such as Parkinson’s disease. The agreement also includes additional collaboration targets leveraging Arrowhead’s proprietary Targeted RNAi Molecule (TRiM™) platform. |
Capital structure and net debt
Retaining a good balance between investment in the business, a strong capital structure, and attractive shareholder returns remains a priority.
During the first nine months of 2025, Novartis repurchased a total of 66.4 million shares for USD 7.5 billion on the SIX Swiss Exchange second trading line. These repurchases included 49.1 million shares (USD 5.4 billion) under the USD 15 billion share buyback (announced in July 2023 and completed in July 2025) and 6.6 million shares (USD 0.8 billion) under the new up-to USD 10 billion share buyback announced in July 2025. In addition, 10.7 million shares (USD 1.3 billion) were repurchased to mitigate anticipated full-year dilution related to the equity-based compensation plans of associates. Further, 1.6 million shares (equity value of USD 0.2 billion) were repurchased from associates. In the same period, 11.7 million shares (equity value of USD 0.9 billion) were delivered to associates related to equity-based compensation plans. Consequently, the total number of shares outstanding decreased by 56.3 million versus December 31, 2024. These treasury share transactions resulted in an equity decrease of USD 6.8 billion and a net cash outflow of USD 7.7 billion.
Net debt increased to USD 20.4 billion at September 30, 2025, compared to USD 16.1 billion at December 31, 2024. The increase was mainly due to the free cash flow of USD 15.9 billion being more than offset by the USD 7.8 billion annual dividend payment, cash outflows for treasury share transactions of USD 7.7 billion and net cash outflow for M&A, intangible assets transactions and other acquisitions of USD 3.7 billion.
As of Q3 2025, the long-term credit rating for the company is Aa3 with Moody’s Ratings and AA- with S&P Global Ratings.
2025 outlook
| Barring unforeseen events; growth vs. prior year in cc | |
| Net sales | Expected to grow high single-digit |
| Core operating income | Expected to grow low-teens |
Foreign exchange impact
If late-October exchange rates prevail for the remainder of 2025, the foreign exchange impact for the year would be neutral to positive 1 percentage point on net sales and negative 2 percentage points on core operating income. The estimated impact of exchange rates on our results is provided monthly on our website.
Key figures1
| Q3 2025 | Q3 2024 | % change | 9M 2025 | 9M 2024 | % change | ||||
| USD m | USD m | USD | cc | USD m | USD m | USD | cc | ||
| Net sales | 13 909 | 12 823 | 8 | 7 | 41 196 | 37 164 | 11 | 11 | |
| Operating income | 4 501 | 3 627 | 24 | 27 | 14 028 | 11 014 | 27 | 31 | |
| As a % of sales | 32.4 | 28.3 | 34.1 | 29.6 | |||||
| Net income | 3 930 | 3 185 | 23 | 25 | 11 563 | 9 119 | 27 | 29 | |
| EPS (USD) | 2.04 | 1.58 | 29 | 31 | 5.94 | 4.50 | 32 | 35 | |
| Net cash flows from operating activities |
6 571 | 6 286 | 5 | 16 880 | 13 426 | 26 | |||
| Non-IFRS measures | |||||||||
| Free cash flow | 6 217 | 5 965 | 4 | 15 941 | 12 618 | 26 | |||
| Core operating income | 5 460 | 5 145 | 6 | 7 | 16 960 | 14 635 | 16 | 18 | |
| As a % of sales | 39.3 | 40.1 | 41.2 | 39.4 | |||||
| Core net income | 4 330 | 4 133 | 5 | 6 | 13 522 | 11 822 | 14 | 17 | |
| Core EPS (USD) | 2.25 | 2.06 | 9 | 10 | 6.94 | 5.83 | 19 | 21 | |
1. Constant currencies (cc), core results and free cash flow are non-IFRS measures. An explanation of non-IFRS measures can be found on page 42 of the Condensed Interim Financial Report. Unless otherwise noted, all growth rates in this Release refer to same period in prior year.
Detailed financial results accompanying this press release are included in the Condensed Interim Financial Report at the link below:
https://ml-eu.globenewswire.com/resource/download/7781ab26-6902-4024-a9c8-49124629eb37/
Disclaimer
This press release contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995, that can generally be identified by words such as “anticipate,” “can,” “will,” “continue,” “ongoing,” “growth,” “launch,” “expect,” “expand,” “deliver,” “accelerate,” “guidance,” “outlook,” “priority,” “potential,” “momentum,” “commitment,” or similar expressions, or by express or implied discussions regarding potential new products, potential new indications for existing products, potential product launches, or regarding potential future revenues from any such products; or regarding results of ongoing clinical trials; or regarding potential future, pending or announced transactions; regarding potential future sales or earnings; or by discussions of strategy, plans, expectations or intentions, including discussions regarding our continued investment into new R&D capabilities and manufacturing; or regarding our capital structure. Such forward-looking statements are based on the current beliefs and expectations of management regarding future events and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. You should not place undue reliance on these statements. There can be no guarantee that the investigational or approved products described in this press release will be submitted or approved for sale or for any additional indications or labeling in any market, or at any particular time. Nor can there be any guarantee that such products will be commercially successful in the future. Neither can there be any guarantee that the expected benefits or synergies from the transactions described in this press release will be achieved in the expected timeframe, or at all. In particular, our expectations could be affected by, among other things: uncertainties concerning global healthcare cost containment, including ongoing government, payer and general public pricing and reimbursement pressures and requirements for increased pricing transparency; uncertainties regarding the success of key products, commercial priorities and strategy; uncertainties in the research and development of new products, including clinical trial results and additional analysis of existing clinical data; our ability to obtain or maintain proprietary intellectual property protection, including the ultimate extent of the impact on Novartis of the loss of patent protection and exclusivity on key products; uncertainties regarding our ability to realize the strategic benefits, operational efficiencies or opportunities expected from our external business opportunities; uncertainties in the development or adoption of potentially transformational digital technologies, including artificial intelligence, and business models; uncertainties surrounding the implementation of our new IT projects and systems; uncertainties regarding potential significant breaches of information security or disruptions of our information technology systems; uncertainties regarding actual or potential legal proceedings, including regulatory actions or delays or government regulation related to the products and pipeline products described in this press release; safety, quality, data integrity, or manufacturing issues; our performance on and ability to comply with environmental, social and governance measures and requirements; major macroeconomic and geo- and socio-political developments, including the impact of any potential tariffs on our products or the impact of war in certain parts of the world; uncertainties regarding future global exchange rates; uncertainties regarding future demand for our products; and other risks and factors referred to in Novartis AG’s most recently filed Form 20-F and in subsequent reports filed with, or furnished to, the US Securities and Exchange Commission. Novartis is providing the information in this press release as of this date and does not undertake any obligation to update any forward-looking statements as a result of new information, future events or otherwise.
All product names appearing in italics are trademarks owned by or licensed to Novartis.
About Novartis
Novartis is an innovative medicines company. Every day, we work to reimagine medicine to improve and extend people’s lives so that patients, healthcare professionals and societies are empowered in the face of serious disease. Our medicines reach nearly 300 million people worldwide.
Reimagine medicine with us: Visit us at https://www.novartis.com and connect with us on LinkedIn, Facebook, X and Instagram.
Novartis will conduct a conference call with investors to discuss this news release today at 14:00 Central European time and 9:00 Eastern Time. A simultaneous webcast of the call for investors and other interested parties may be accessed by visiting the Novartis website. A replay will be available after the live webcast by visiting https://www.novartis.com/investors/event-calendar.
Detailed financial results accompanying this press release are included in the Condensed Interim Financial Report at the link below. Additional information is provided on our business and pipeline of selected compounds in late-stage development. A copy of today’s earnings call presentation can be found at https://www.novartis.com/investors/event-calendar.
| Important dates | |
| October 30, 2025 | Immunology pipeline event at ACR (virtual) |
| November 19-20, 2025 | Meet Novartis Management 2025 (London, UK) |
| December 1, 2025 | Social Impact & Sustainability annual investor event (virtual) |
| February 4, 2026 | Fourth quarter & full year 2025 results |
# # #
Please find full media release in English attached and on the following link:
Media Release (PDF)
Further language versions are available through the following links:
German version is available through the following link:
Medienmitteilung (PDF)
Two-and-a-half years ago, Léna Descottes (29) thought her time in Ireland was over. Despite being a traditional-music performer who had played in venues across the country, she had spent the previous six months sleeping primarily in her car….
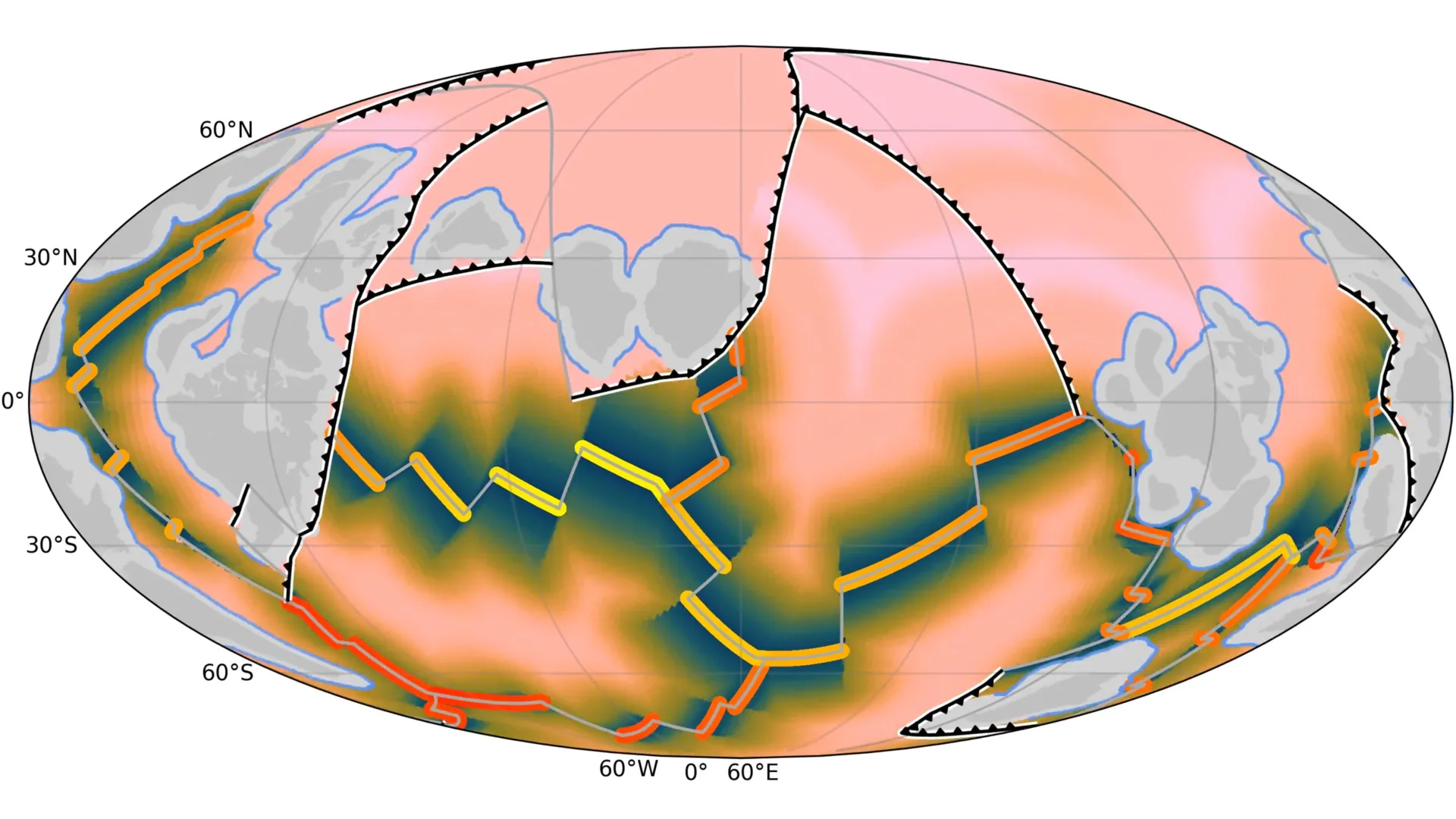
Scientists from the University of Sydney and the University of Adelaide have uncovered how the breakup of an ancient supercontinent about 1.5 billion years ago reshaped Earth’s surface and set the stage for the rise of complex life.
“Our approach…

Scientists from the University of Sydney and the University of Adelaide have uncovered how the breakup of an ancient supercontinent about 1.5 billion years ago reshaped Earth’s surface and set the stage for the rise of complex life.
“Our approach…

Shaimaa Khalil
Tokyo correspondent
So far, one remark stands out. President Trump is affording Ms Takaichi a lot of good will.
“I want to just let you know – any time you have any question, any doubt, anything you want, any favors you need, anything I can do to help Japan, we will be there.”
It’s a critical moment for Japan and for its new PM Sanae Takaichi – a defining early test of leadership. In her first face-to-face meeting with President Trump, she described him as a partner in a new golden age and praised his role in Middle East peace.
President Trump praised Japan as “a great ally” and said he’ll be there to help Japan whenever needed. But he’s also pushing hard on trade and security, leaving Tokyo backed into a corner.
Trump wants more US access to Japan’s markets – especially in cars, agriculture, and technology. He’s pressing Japan to buy more American rice and soybeans, and to open its market to US vehicles.
Tokyo, heavily reliant on exports, can’t afford a tariff fight – especially when it comes to its auto industry.
But Takaichi also needs to protect domestic industries and doesn’t want to anger crucial interest groups like the powerful farming lobby.
For now, the tone is friendly. But there’s real pressure on Tokyo to deliver on agreements with little room to manoeuvre.

Every second matters when a haemorrhage or clot disrupts blood flow to the brain and cells up there begin to die.
Known as a stroke, it will happen to one in four of us.
It is then a race against time, in which both the public and health…


Significant insider control over Pan-United implies vested interests in company growth
The top 3 shareholders own 64% of the company
Recent sales by insiders
Trump has pledged to “unleash” American oil and gas and these 15 US stocks have developments that are poised to benefit.
If you want to know who really controls Pan-United Corporation Ltd (SGX:P52), then you’ll have to look at the makeup of its share registry. And the group that holds the biggest piece of the pie are individual insiders with 72% ownership. In other words, the group stands to gain the most (or lose the most) from their investment into the company.
And insiders own the top position in the company’s share registry despite recent sales.
Let’s take a closer look to see what the different types of shareholders can tell us about Pan-United.
View our latest analysis for Pan-United
Small companies that are not very actively traded often lack institutional investors, but it’s less common to see large companies without them.
There could be various reasons why no institutions own shares in a company. Typically, small, newly listed companies don’t attract much attention from fund managers, because it would not be possible for large fund managers to build a meaningful position in the company. It is also possible that fund managers don’t own the stock because they aren’t convinced it will perform well. Pan-United might not have the sort of past performance institutions are looking for, or perhaps they simply have not studied the business closely.
Hedge funds don’t have many shares in Pan-United. The company’s largest shareholder is Han Whatt Ng, with ownership of 23%. The second and third largest shareholders are Bee Kiok Ng and Bee Bee Ng, with an equal amount of shares to their name at 21%. Bee Bee Ng, who is the third-largest shareholder, also happens to hold the title of Chairman of the Board.
To make our study more interesting, we found that the top 3 shareholders have a majority ownership in the company, meaning that they are powerful enough to influence the decisions of the company.
While it makes sense to study institutional ownership data for a company, it also makes sense to study analyst sentiments to know which way the wind is blowing. There are plenty of analysts covering the stock, so it might be worth seeing what they are forecasting, too.
The definition of company insiders can be subjective and does vary between jurisdictions. Our data reflects individual insiders, capturing board members at the very least. Company management run the business, but the CEO will answer to the board, even if he or she is a member of it.