Battlefield 6’s arrival in the cloud marks a milestone for mobile and low-end hardware gamers, delivering the series’ signature large-scale battles wherever you are.
GeForce NOW brings Battlefield 6 to the cloud at launch
NVIDIA announced…

Battlefield 6’s arrival in the cloud marks a milestone for mobile and low-end hardware gamers, delivering the series’ signature large-scale battles wherever you are.
NVIDIA announced…

Bitcoin trades near $121.6K, testing major resistance at $126K. Analysts watch for a breakout or correction as futures open interest stays high.
Bitcoin is trading near $121,600 after reaching a new all-time high above $126,000 earlier this week. The asset has slowed and is now moving sideways just below a major diagonal resistance that has capped the price for several months. Analysts are watching to see whether Bitcoin can break through or if it will be rejected once again.
Meanwhile, the market is holding near a key level while open interest remains high in the futures market.
Bitcoin is facing resistance at a trendline that has held firm since earlier cycle tops. The latest weekly candle shows another rejection near $126,000, followed by a pullback of around 5.5%. Earlier this year, similar rejections led to drops of nearly 10% and close to 30%.
The difference this time is the size of the pullbacks. Each rejection has been smaller than the last. Rekt Capital pointed out that “this trendline looks to be a weakening point of rejection,” suggesting sellers may be losing strength at this level.
On the lower timeframes, Bitcoin is holding just above $121,000. This zone is marked by a previous support level and lines up with a Fibonacci retracement near $119,550. Daan Crypto Trades noted that this is the area to watch.
$BTC Not here to overcomplicate things.
We saw a sharp impulse up and an all time high sweep.
Currently price is consolidating beneath this diagonal resistance. If that’s broken, I am expecting this to move to new highs pretty quickly.
Key area to hold below is ~$120K. If… pic.twitter.com/MK7gYzINWJ
— Daan Crypto Trades (@DaanCrypto) October 10, 2025
The asset is currently consolidating in a tight range. Volume has slowed, which often happens before a larger move. The trend remains bullish overall. A confirmed higher low would support another leg higher, especially if resistance breaks.
While short-term sentiment remains positive, some market participants are still cautious. Analyst Ali Martinez shared a scenario where Bitcoin gets rejected at $124,000 and begins a correction toward $96,000. If that level fails, the next major support is around $70,000.
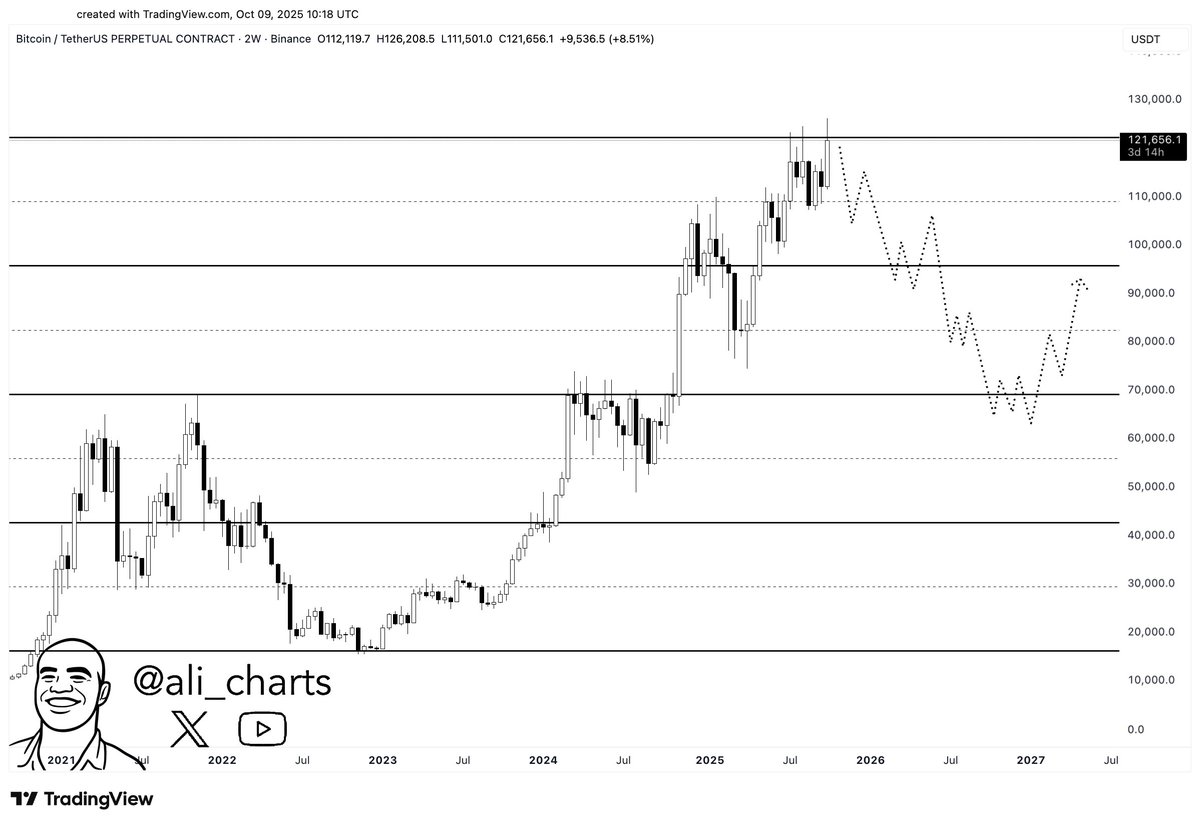
The projected path shows a slow recovery beginning in late 2026, with Bitcoin climbing back toward $90,000. This scenario reflects a broader retracement, not a trend reversal, and suggests long-term structure could still remain intact.
Data from Glassnode shows that futures open interest is still elevated. This means that many traders are positioned on both the long and short sides. Sharp moves in either direction have triggered liquidations. Glassnode said the market is undergoing “a leverage reset” as volatility flushes out excess positions.
With Bitcoin sitting just below resistance and leverage still high, traders are watching closely. A move above the current range could open the way toward $130,000. If resistance holds, another pullback could extend the current consolidation.
LIMITED OFFER for CryptoPotato readers at Bybit: Use this link to register and open a $500 FREE position on any coin!

Paul KirbyEurope digital editor
 AFP
AFPPresident Emmanuel Macron has asked Sébastien Lecornu to return as French prime minister only four days after he…

Science and maintenance work continued this week aboard the International Space Station, but updates regarding those activities have been all but halted by the U.S. government shutdown. Japanese astronaut Kimiya Yui’s social media posts were the…
AstraZeneca today announces a historic agreement with President Donald J. Trump’s administration to lower the cost of prescription medicines for American patients while preserving America’s cutting-edge biopharmaceutical innovation.
At a landmark event at the White House, AstraZeneca CEO Pascal Soriot joined President Trump and members of his Administration to confirm the Company voluntarily met all requests set out in the President’s 31 July letter. The Company agrees to a range of measures which will enable American patients to access medicines at prices that are equalized with those available in wealthy countries.
As part of the agreement, AstraZeneca will provide Direct-to-Consumer (DTC) sales to eligible patients with prescriptions for chronic diseases at a discount of up to 80% off list prices. AstraZeneca will participate in the TrumpRx.gov direct purchasing platform, which will allow patients to purchase medicines at a reduced cash price from AstraZeneca.
AstraZeneca has also reached an agreement with the US Department of Commerce to delay Section 232 tariffs for three years, enabling the Company to fully onshore medicines manufacturing so that all of its medicines sold in America are made in America. This will be achieved through the Company’s recently announced $50 billion investment in US medicines manufacturing and R&D over the next five years to help deliver $80 billion in Total Revenue by 2030, 50% of which is expected to be generated in the US.
Pascal Soriot, Chief Executive Officer, AstraZeneca, said: “Every year AstraZeneca treats millions of Americans living with cancer and chronic diseases and, as a result of today’s agreement, many patients will access life-changing medicines at lower prices. This new approach also helps safeguard America’s pioneering role as a global powerhouse in innovation and developing the next generation of medicines. It is now essential other wealthy countries step up their contribution to fund innovation.”
AstraZeneca’s commitment to the US and American patients is further reflected in the Company’s largest single investment in a manufacturing facility to date, where the Company broke ground yesterday in Virginia. This facility will support AstraZeneca’s weight management and metabolic portfolio and our leading antibody drug conjugate cancer pipeline. Additionally, a newly expanded manufacturing facility in Coppell, Texas, will officially open next week. Looking ahead, AstraZeneca will open a cell therapy manufacturing facility in Rockville, Maryland early next year and its second major R&D centre in Cambridge, Massachusetts will open in late 2026.
The US is AstraZeneca’s largest market by sales and is also home to 19 R&D, manufacturing and commercial sites. The Company’s US workforce exceeds more than 25,000 people and supports more than 100,000 jobs overall across the country. In 2025, AstraZeneca created approximately $20 billion of overall value to the American economy.
Notes
AstraZeneca’s agreement with US Government
This is the second agreement that a pharmaceutical company has made with the US Department of Health and Human Services to lower the cost of medicines for American patients in the past two weeks. Specific terms of this agreement remain confidential.
AstraZeneca
AstraZeneca (LSE/STO/Nasdaq: AZN) is a global, science-led biopharmaceutical company that focuses on the discovery, development, and commercialisation of prescription medicines in Oncology, Rare Diseases, and BioPharmaceuticals, including Cardiovascular, Renal & Metabolism, and Respiratory & Immunology. Based in Cambridge, UK, AstraZeneca’s innovative medicines are sold in more than 125 countries and used by millions of patients worldwide. Please visit astrazeneca.com and follow the Company on social media @AstraZeneca. The contents of AstraZeneca’s website do not form part of this document and no one should rely on such websites or the contents thereof in reading this document.
Contacts
For details on how to contact the Investor Relations Team, please click here. For Media contacts, click here.

An experimental gene therapy from Sarepta Therapeutics increased levels of the gene missing in an ultra-rare form of muscular dystrophy, according to data the company presented Friday.
The company has said it plans to file for approval in the disease, known as limb-girdle muscular dystrophy (LGMD) 2E. That would make it the first approved treatment in LGMD, a broad collection of highly rare diseases that can deprive patients of the ability to walk and in some cases shorten life. But it is likely to face a significant uphill battle.
The LGMD 2E therapy relies on the same gene-ferrying virus that Sarepta uses in its other treatments, including its approved gene therapy for Duchenne muscular dystrophy, Elevidys, and experimental gene therapies for several other LGMD subtypes.
Already have an account? Log in
View All Plans

Southwest of Black Mesa and north of Cerro Verde, Cibola County, New Mexico, 2015. License: CC0.
Notes
1
Walter De Maria, “The Lightning Field,” Artforum 18, no. 8 (April 1980). All section headings in…
Instagram head Adam Mosseri has announced that as part of a test, some Instagram users will be able to try a new menu bar in the app with a different arrangement of tabs. Notably, the new menu bar has dedicated tabs for both Reels and DMs, two of…

Two greenish-hued comets are swinging through the inner solar system this fall, offering a rare chance to spot them in the coming weeks.
The comets, named C/2025 A6 (Lemmon) and C/2025 R2 (SWAN), are visible from the Northern Hemisphere now as…
LAHORE: Violent clashes erupted Friday between police and Islamists in Pakistan’s eastern city of Lahore after security forces tried to stop thousands of demonstrators from…