Casio Computer Co., Ltd. announced today the release of a new addition to the G-STEEL line of G-SHOCK brand of shock-resistant watches, which combines signature toughness with a sleek metal exterior. The new GST-B1000D features a minimalist…
Blog
-

TV tonight: heart-warming nostalgia with Tom Jones | Television
In My Own Words: Tom Jones
10.40pm, BBC One
A lovely, intimate trip down memory lane as the Welshman with the voice watches back smile-raising footage of his life. From working-class life in Pontypridd to breakout hit It’s Not Unusual, his first…Continue Reading
-
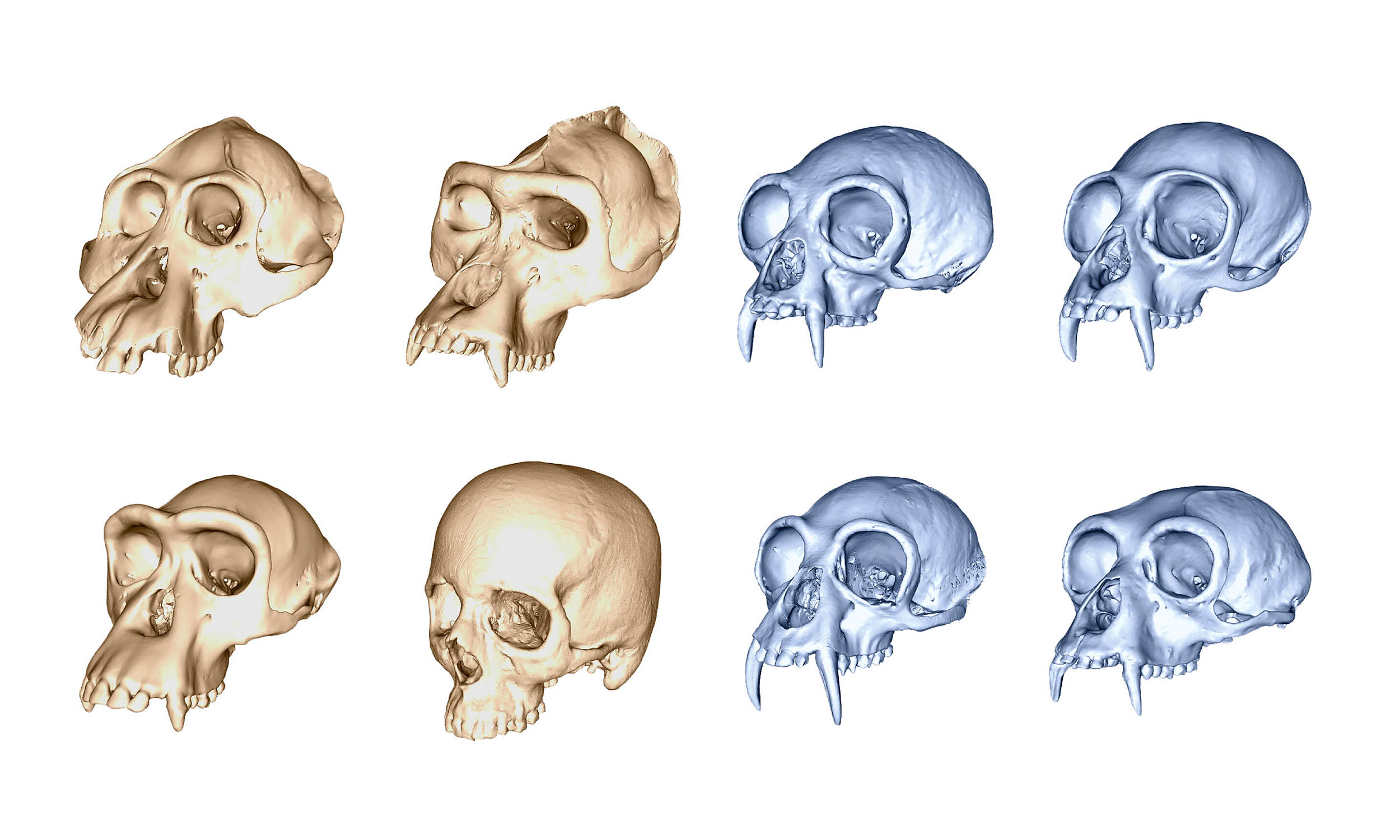
3D skull study shows humans evolved faster than any other ape
Every human face hides a long evolutionary story. Behind the curve of the skull and the flatness of the cheeks lies a record of rapid change.
A new study from University College London (UCL) shows that our skulls didn’t just evolve – they…
Continue Reading
-

Q3 2025: Air Liquide continues to combine sales growth with commercial successes to shape the future
Commenting on sales in the third quarter of 2025, François Jackow, Chief Executive Officer of the Air Liquide Group, stated:
“Air Liquide delivered another very solid performance, continuing its profitable growth trajectory. In line with the first half of the year and despite a difficult environment, our sales continue to increase, once again demonstrating the strength of our business model.
Amounting to nearly 6.6 billion euros at September 30, 2025, our revenue was up +1.9% on a comparable basis (‑2.4% on a reported basis, reflecting a negative currency impact and lower energy prices, which were passed on to our customers). The Gas and Services businesses, which accounts for 97% of the Group’s revenue, increased by +1.9% on a comparable basis, to reach 6,386 million euros. In an uncertain industrial environment, Healthcare and Industrial Merchant were growth drivers, up sharply by +5% and +3%, respectively, on a comparable basis. Geographically, the Americas stood out in particular with a +5% growth.
Air Liquide also continued to improve its performance. At the end of September, the Group’s efficiencies were at a record high of +23%. We also continued the dynamic management of our business portfolio, while adjusting our prices in Industrial Merchant thanks to our ability to create value for our customers. Our cash flow is very solid, increasing by +7% excluding currency impact.
Paving the way for future growth, our investment momentum is particularly strong. Well diversified, our investment backlog is again at a record level of nearly 5 billion euros in this third quarter. Our investment decisions amounted to 0.9 billion euros, with major industrial projects supporting the energy transition, such as ELYgator, our 200 MW electrolyzer in the Netherlands, but also in Electronics & Semiconductors. New state-of-the-art industrial gas production units will be built in Dresden, Germany for a major player in this industry, driven by AI and sovereignty needs.
In addition, our outlook includes our planned acquisition of DIG Airgas, a leading industrial gas company in South Korea. Beyond the dynamism and innovation that characterize the country’s economy, this transaction will quickly create value, thanks to the high complementarity between our businesses and the nearly 20 projects already secured. It will therefore contribute to our net profit the year following its integration.
In this context, Air Liquide is very confident in its ability to further increase its operating margin[1] and to deliver recurring net profit[2] growth, at constant exchange rates in 2025. The Group also maintains its ambition to increase its operating margin by +460 basis points cumulated over five years to end-2026[1].”
Highlights
-
Signature of an agreement to acquire DIG Airgas, a leader in industrial gases in South Korea. This major strategic acquisition, the largest since the acquisition of Airgas in the United States in 2016, aims to significantly strengthen the Group’s position in the South Korean market. This is a growth opportunity in a country known worldwide for its dynamic economy and fast-growing business sectors such as Electronics, clean energy, mobility and biopharma.
-
Hydrogen
-
In the United States, on the coast of the Gulf of Mexico, development of hydrogen production businesses. Air Liquide will build on its existing infrastructure with close to 50 million US dollars in targeted investments to supply two of the country’s largest refiners.
-
-
Electronics
-
In Germany, more than 250 million euros invested to build new state-of-the-art industrial gas production units for a major customer in the semiconductor industry located in SiliconSaxony.
-
In the United States, a 50 million US dollar investment to support the growth of the semiconductor industry. An additional ultra-pure gas production plant will be built on the site of one of the largest manufacturers in the world for advanced chip design.
-
Due to the acceleration of demand for advanced electronic components, two new contracts were signed in Singapore. The Group will build, own and operate new state-of-the-art industrial gas production facilities that will support the expansion of a major manufacturer, for a total investment of 130 million euros.
-
-
Healthcare
-
Award of a major five-year contract with the Community of Madrid to provide home care for 70,000 patients suffering from respiratory diseases. This success consolidates Air Liquide’s leading position in the Spanish market.
-
Group revenue[3] stood at 6,599 million euros in the 3rd quarter 2025, a comparable growth of +1.9% compared to the 3rd quarter 2024. This growth continued in line with the 1st half of the year, benefiting from the resilience of the business portfolio in a complex environment. The Group’s published revenue was down -2.4%, impacted by an unfavorable currency impact (-4.2%), with the energy impact being neutral (-0.1%). There was no significant scope impact in the 3rd quarter 2025.
Gas & Services revenue in the 3rd quarter 2025 reached 6,386 million euros, up by +1.9% on a comparable basis.
Sales growth for the Industrial Merchant business stood at +2.7%[4] in the 3rd quarter: it benefited from a price effect of +3.1% which continues to strengthen, and improving volumes, particularly for Hardgoods, supported by the consolidation of bolt-on acquisitions. Revenue for Large Industries was stable (-0.2%[4]), with the contribution from the start-up and ramp-up of units offsetting weak demand, particularly in Europe and Asia. The slight decline in Electronics sales (-0.9%) does not reflect the dynamic growth of the business excluding Equipment & Installation sales (+5.9%). The latter are indeed more cyclical and are normalizing after reaching a record level in 2024. Finally, the Healthcare business, whose growth is disconnected from industrial trends, posted sustained revenue growth (+4.9%), particularly in Home Healthcare and Specialty Ingredients.
-
Gas & Services revenue in the Americas stood at 2,548 million euros in the 3rd quarter 2025, up by +4.8%[5]. Sales growth for Large Industries (+5.2%[4]) benefited from recent start-ups of new production units and resilient demand. In Industrial Merchant, revenue increased by +4.7%(4), supported by a very solid price effect of +4.5%, resilient gas volumes, and by the contribution of bolt-on acquisitions, while volumes for hardgoods are improving but remain down compared to the 3rd quarter 2024. Strong sales growth in Healthcare (+9.3%) was mainly driven by a strong price effect in the Medical Gases business in the United States and by the development of Home Healthcare in Latin America. In Electronics (-3.8%), the significant decline in Equipment & Installation sales masked the dynamic growth of the rest of the business (+5.6%).
-
Revenue in the Europe Middle East & Africa region stood at 2,584 million euros, up slightly by +0.4% compared to the 3rd quarter 2024. In Large Industries (-2.0%), sales were mainly impacted in Germany by weak demand and a customer shutdown for force majeure, and in Benelux by lower sales from cogeneration units. Sales were stable in Industrial Merchant (0.0%), supported by a solid price effect and resilient gas volumes, with the exception of Helium and liquid CO2. Sales growth remained strong (+4.3%) in Healthcare, particularly in Home Healthcare and Specialty ingredients.
-
Revenue in the Asia-Pacific region stood at 1,255 million euros in the 3rd quarter 2025, down -0.8% compared to the 3rd quarter 2024. In Large Industries, sales were slightly down (-0.6%), with the contribution from recent start-ups of new production units partially offsetting overall weak demand in the region. Industrial Merchant revenue (-0.8%) was impacted by the marked decrease in helium sales in China and by weak revenue in the rest of the zone, despite otherwise growing sales in China. The stability (+0.2%) of sales in Electronics masked dynamic growth of the business excluding Equipment & Installation sales (+6.3%), with in particular the start-up of seven new production units in Asia since the beginning of the year.
Engineering & Technologies[6] revenue stood at 212 million euros in the 3rd quarter 2025, a comparable growth of +1.7%.
Industrial and financial investment decisions stood at 924 million euros in the 3rd quarter 2025 and 3.2 billion euros at the end of September. The investment backlog remains above 4.0 billion euros and reaches a new record at 4.9 billion euros, up from 4.6 billion euros at the end of June 2025.
The additional contribution to sales from ramp-ups and start-ups of units amounted to 233 million euros at the end of the 3rd quarter. For the full year 2025, it is expected to be between 310 and 340 million euros.
The 12-month portfolio of investment opportunities remained at the high level of 4.1 billion euros at the end of September 2025. The total portfolio of opportunities, also including opportunities beyond 12 months, was stable and exceeded 10 billion euros.
Efficiencies reached 163 million euros in the 3rd quarter. They amounted to 434 million euros over the first 9 months of the year, a strong increase of +22.9% compared to the same period in 2024.
Cash flow from operating activities before changes in working capital stood at 4,947 million euros at the end of September, up by +6.8% excluding currency impact.
Net debt stood at 9,317 million euros at the end of September, down by 477 million euros compared to 9,794 million euros at June 30, 2025.
Footnotes
- Excluding energy passthrough impact. ↑
- Recurring net profit excluding exceptional and significant transactions that have no impact on the operating income recurring. ↑
- Unless otherwise specified, the revenue variations are all variations on a comparable basis, excluding currency, energy (natural gas and electricity) and significant scope impacts. ↑
- Excluding an internal transfer of assets between Large Industries and Industrial Merchant in the United States ↑
- Includes Argentina’s contribution of +0.6%, down sharply compared to 2024. ↑
- This comparable growth excludes the scope impact related to the internal transfer of some GM&T activities to Industrial Merchant in the 1st quarter 2025. See appendix. ↑
Continue Reading
-
-

‘I like to work out killer as plot unravels’
Catherine LystBBC Scotland
 ITV Studios
ITV StudiosDI Ruth Calder (Ashley Jensen) and DI Alison “Tosh” McIntosh (Alison O’Donnell) play two detectives trying to find a murderer in a remote village Shetland star Ashley Jensen has revealed she doesn’t like to…
Continue Reading
-

Bird flu confirmed in Wellingborough after swan deaths reported
Kris HollandNorthamptonshire
 Getty Images
Getty ImagesDefra reported that avian flu was found to be present in four mute swans Avian flu has been confirmed in six wild birds in Northamptonshire, according to government data.
Figures issued by the Department for…
Continue Reading
-

Lauri Markkanen scores 51 points, 1st Jazz player to top 50 since Karl Malone
Lauri Markkanen erupts for career-high 51 points in the Jazz’s victory over the Suns on Monday.
SALT LAKE CITY (AP) — The Utah Jazz played 2,155 regular season games since Karl Malone scored 56 points in awin over Golden State on April 7,…
Continue Reading
-

The next iPad Pro will come with M6 chip, vapor chamber cooling
In the latest episode of Chinese-Exclusive Smartphone Launch: Season 10/25, Redmi (or Xiaomi) unsurprisingly launches their latest and greatest flagship killer, the K90 Pro Max.
First “Pro Max” Midranger
Much like the its…
Continue Reading
-

Novartis delivers solid sales and core operating income growth with strong pipeline progress in Q3; reaffirms FY 2025 guidance
Ad hoc announcement pursuant to Art. 53 LR
- Q3 net sales grew +7% (cc1, +8% USD) and core operating income1 grew +7% (cc, +6% USD)
- Sales growth was driven by continued strong execution on priority brands including Kisqali (+68% cc), Kesimpta (+44% cc), Pluvicto (+45% cc) and Scemblix (+95% cc)
- Core operating income margin1 was stable (cc) at 39.3% despite increasing generic impact
- Q3 operating income grew +27% (cc, +24% USD); net income rose +25% (cc, +23% USD)
- Q3 core EPS1 grew +10% (cc, +9% USD) to USD 2.25
- Q3 free cash flow1 was USD 6.2 billion (+4% USD) driven by higher net cash flows from operating activities
- Strong nine months performance with net sales up +11% (cc, +11% USD) and core operating income up +18% (cc, +16% USD)
- Q3 selected innovation milestones:
- Rhapsido FDA approval as the only oral, targeted BTK inhibitor for CSU
- Ianalumab positive replicate Phase III readouts in Sjogren’s disease
- Pluvicto positive Phase III PSMAddition data at ESMO
- Scemblix positive CHMP opinion for all lines of CML treatment
- Cosentyx positive Phase III readout in PMR
- Fabhalta positive Phase III eGFR readout in IgA nephropathy
- Full-year 2025 guidance2 reaffirmed
- Sales expected to grow high single-digit
- Core operating income expected to grow low-teens
1. Constant currencies (cc), core results and free cash flow are non-IFRS measures. An explanation of non-IFRS measures can be found on page 42 of the Condensed Interim Financial Report. Unless otherwise noted, all growth rates in this Release refer to same period in prior year. 2. Please see detailed guidance assumptions on page 7.
Basel, October 28, 2025 – Commenting on Q3 2025 results, Vas Narasimhan, CEO of Novartis, said:
“Novartis delivered solid financial performance in Q3, more than offsetting the impact of increasing generic erosion in the US. Our key growth drivers performed well, including Kisqali, Kesimpta, Pluvicto and Scemblix. Importantly, we achieved FDA approval for Rhapsido in CSU and positive Phase III readouts for ianalumab in Sjogren’s disease – two assets with pipeline-in-a-pill potential that could underpin our growth through 2030 and beyond. In addition, we completed several deals in the quarter to further strengthen our pipeline in core therapeutic areas. We remain well on track to achieve our guidance for 2025 and over the mid-term.”Key figures Q3 2025 Q3 2024 % change 9M 2025 9M 2024 % change USD m USD m USD cc USD m USD m USD cc Net sales 13 909 12 823 8 7 41 196 37 164 11 11 Operating income 4 501 3 627 24 27 14 028 11 014 27 31 Net income 3 930 3 185 23 25 11 563 9 119 27 29 EPS (USD) 2.04 1.58 29 31 5.94 4.50 32 35 Free cash flow 6 217 5 965 4 15 941 12 618 26 Core operating income 5 460 5 145 6 7 16 960 14 635 16 18 Core net income 4 330 4 133 5 6 13 522 11 822 14 17 Core EPS (USD) 2.25 2.06 9 10 6.94 5.83 19 21 Strategy
Our focus
Novartis is a “pure-play” innovative medicines company. We have a clear focus on four core therapeutic areas (cardiovascular-renal-metabolic, immunology, neuroscience and oncology), with multiple significant in-market and pipeline assets in each of these areas, that address high disease burden and have substantial growth potential. In addition to two established technology platforms (chemistry and biotherapeutics), three emerging platforms (gene & cell therapy, radioligand therapy and xRNA) are being prioritized for continued investment into new R&D capabilities and manufacturing scale. Geographically, we are focused on growing in our priority geographies – the US, China, Germany and Japan.
Our priorities
- Accelerate growth: Renewed attention to deliver high-value medicines (NMEs) and focus on launch excellence, with a rich pipeline across our core therapeutic areas.
- Deliver returns: Continuing to embed operational excellence and deliver improved financials. Novartis remains disciplined and shareholder-focused in our approach to capital allocation, with substantial cash generation and a strong capital structure supporting continued flexibility.
- Strengthen foundations: Unleashing the power of our people, scaling data science and technology and continuing to build trust with society.
Financials
Third quarter
Net sales were USD 13.9 billion (+8%, +7% cc), with volume contributing 16 percentage points to growth. Generic competition had a negative impact of 7 percentage points, driven by Promacta, Tasigna and Entresto generics in the US. Pricing had a negative impact of 2 percentage points, driven by revenue deduction adjustments mainly in the US. Currency had a positive impact of 1 percentage point.
Operating income was USD 4.5 billion (+24%, +27% cc), mainly driven by higher net sales and lower impairments, partly offset by higher R&D investments.
Net income was USD 3.9 billion (+23%, +25% cc), mainly driven by higher operating income. EPS was USD 2.04 (+29%, +31% cc), benefiting from the lower weighted average number of shares outstanding.
Core operating income was USD 5.5 billion (+6%, +7% cc), mainly driven by higher net sales, partly offset by higher R&D investments. Core operating income margin was 39.3% of net sales (-0.8 percentage points, stable in cc).
Core net income was USD 4.3 billion (+5%, +6% cc), mainly due to higher core operating income, partly offset by other core financial income and expense. Core EPS was USD 2.25 (+9%, +10% cc), benefiting from the lower weighted average number of shares outstanding.
Free cash flow amounted to USD 6.2 billion (+4% USD), compared with USD 6.0 billion in the prior-year quarter, driven by higher net cash flows from operating activities.
Nine months
Net sales were USD 41.2 billion (+11%, +11% cc), with volume contributing 14 percentage points to growth. Generic competition had a negative impact of 3 percentage points, while pricing and currency had no impact.
Operating income was USD 14.0 billion (+27%, +31% cc), mainly driven by higher net sales and lower impairments, partly offset by higher investments behind priority brands and launches.
Net income was USD 11.6 billion (+27%, +29% cc), mainly driven by higher operating income. EPS was USD 5.94 (+32%, +35% cc), benefiting from the lower weighted average number of shares outstanding.
Core operating income was USD 17.0 billion (+16%, +18% cc), mainly driven by higher net sales, partly offset by higher investments behind priority brands and launches. Core operating income margin was 41.2% of net sales, increasing 1.8 percentage points (2.5 percentage points cc).
Core net income was USD 13.5 billion (+14%, +17% cc), mainly due to higher core operating income. Core EPS was USD 6.94 (+19%, +21% cc), benefiting from the lower weighted average number of shares outstanding.
Free cash flow amounted to USD 15.9 billion (+26% USD), compared with USD 12.6 billion in the prior-year period, driven by higher net cash flows from operating activities.
Q3 priority brands
Underpinning our financial results in the quarter is a continued focus on key growth drivers (ranked in order of contribution to Q3 growth) including:
Kisqali (USD 1 329 million, +68% cc) sales grew strongly across all regions, including +91% growth in the US with strong momentum from the recently launched early breast cancer indication as well as continued share gains in metastatic breast cancer. Kesimpta (USD 1 222 million, +44% cc) sales grew across all regions driven by increased demand and strong access. Pluvicto (USD 564 million, +45% cc) showed sustained demand growth in the US following the pre-taxane metastatic castration-resistant prostate cancer (mCRPC) approval, as well as continued access expansion ex-US in the post-taxane mCRPC setting, with 25 countries now approved including Japan. Scemblix (USD 358 million, +95% cc) sales grew across all regions, demonstrating the continued high unmet need in CML, with strong momentum from the early-line indication in the US and Japan. Leqvio (USD 308 million, +54% cc) continued steady growth across all regions, with a focus on increasing account and patient adoption, and continuing medical education. Fabhalta (USD 149 million, +236% cc) sales grew, reflecting market share gains in PNH globally and continued launch progress in IgAN and C3G in the US. Lutathera (USD 213 million, +11% cc) sales grew mainly in the US, Japan and Europe due to increased demand and earlier-line adoption. Cosentyx (USD 1 698 million, -1% cc) sales were broadly stable, as strong volume growth in the US was partially offset by higher revenue deductions, and ex-US declined due to a one-time price effect in the prior year. Novartis remains confident in Cosentyx USD 8 billion+ peak sales guidance. Zolgensma (USD 301 million, -5% cc) sales declined reflecting a lower incidence of SMA compared to prior year.
Net sales of the top 20 brands in the third quarter and nine monthsQ3 2025 % change 9M 2025 % change USD m USD cc USD m USD cc Entresto 1 877 1 -1 6 495 15 15 Cosentyx
– excl. revenue deduction adjust.*1 698 0
5-1
44 861 7
97
9Kisqali 1 329 69 68 3 462 62 63 Kesimpta 1 222 46 44 3 198 41 40 Tafinlar + Mekinist 550 3 1 1 675 9 9 Jakavi 539 8 4 1 555 7 6 Promacta/Revolade 362 -36 -38 1 410 -14 -14 Pluvicto 564 46 45 1 389 33 33 Ilaris 473 27 26 1 369 25 24 Xolair 440 5 3 1 339 8 8 Tasigna 221 -47 -48 925 -27 -26 Zolgensma 301 -2 -5 925 -3 -4 SandostatinGroup 302 -1 -1 922 -5 -5 Scemblix 358 97 95 894 85 84 Leqvio 308 56 54 863 63 61 Lutathera 213 12 11 613 15 14 ExforgeGroup 176 1 0 546 0 2 Lucentis 148 -40 -42 510 -39 -39 DiovanGroup 143 -5 -5 447 -1 0 GalvusGroup 126 -21 -20 373 -19 -16 Top 20 brands total 11 350 10 9 33 771 14 14 *Sales growth impacted by a one-time revenue deduction adjustment in the US
R&D update – key developments from the third quarter
New approvals
Rhapsido
(remibrutinib)Rhapsido was approved by the FDA as an oral treatment for adult patients with chronic spontaneous urticaria (CSU) who remain symptomatic despite H1 antihistamine treatment. It is the first FDA-approved Bruton’s tyrosine kinase inhibitor (BTKi) for CSU. Remibrutinib is also in Phase III development for chronic inducible urticaria, hidradenitis suppurativa and food allergy, as well as multiple sclerosis and myasthenia gravis. Regulatory updates
Scemblix (asciminib) The CHMP of the EMA adopted a positive opinion and recommended granting marketing authorization for Scemblix for the treatment of adult patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase (Ph+ CML-CP) in all lines of treatment.
Results from ongoing trials and other highlightsIanalumab
(VAY736)The Phase III NEPTUNUS-1 and -2 trials evaluating ianalumab in adults with active Sjögren’s disease met their primary endpoint, showing statistically significant improvements in disease activity as measured by a reduction in ESSDAI compared to placebo. Ianalumab was well tolerated and demonstrated a favorable safety profile, supporting its potential to become the first targeted treatment for this chronic autoimmune disease. Novartis plans to submit ianalumab to health authorities globally and was granted Fast Track Designation by the FDA. In the Phase III VAYHIT2 trial, ianalumab plus eltrombopag significantly extended the time to treatment failure compared to placebo plus eltrombopag in adult patients with primary immune thrombocytopenia (ITP), previously treated with corticosteroids. The safety profile was consistent with previous studies. Data will be presented at an upcoming medical meeting and included in regulatory submissions in 2027.
Ianalumab is also in Phase III development for systemic lupus erythematosus, lupus nephritis and warm autoimmune hemolytic anemia.
Pluvicto
(lutetium Lu177
vipivotide
tetraxetan)In the Phase III PSMAddition trial, Pluvicto plus standard-of-care (SoC) reduced risk of progression or death by 28% versus SoC alone, with a positive trend in overall survival in patients with PSMA+ metastatic hormone-sensitive prostate cancer (mHSPC). Safety remained consistent with PSMAfore and VISION trials. Data presented at ESMO. Kisqali
(ribociclib)The five-year analysis of the pivotal Phase III NATALEE trial in the broadest population of high-risk stage II and III HR+/HER2- early breast cancer (eBC) showed the addition of Kisqali to endocrine therapy (ET) reduced the risk of recurrence by 28.4% compared to ET alone. Data also showed a 29.1% risk reduction in distant disease-free survival, a positive trend in overall survival, and no new safety signals. Data presented at ESMO. Cosentyx
(secukinumab)The Phase III REPLENISH study met its primary endpoint, with Cosentyx demonstrating statistically significant and clinically meaningful sustained remission compared to placebo at week 52 in adults with relapsing polymyalgia rheumatica (PMR). Full data will be presented at an upcoming medical congress and submitted to health authorities in 2026. Fabhalta
(iptacopan)In the Phase III APPLAUSE-IgAN final analysis, Fabhalta demonstrated statistically significant, clinically meaningful superiority compared to placebo in slowing IgAN progression measured by annualized total slope of estimated glomerular filtration rate (eGFR) decline over two years. Full data will be presented at future medical meetings and included in regulatory submissions in 2026. Leqvio
(Inclisiran)In the Phase IV V-DIFFERENCE study, 85% of patients with hypercholesterolemia who had not reached guideline-recommended LDL-C targets despite optimized lipid-lowering therapy (LLT) achieved their goals with Leqvio plus LLT, versus 31% with placebo plus LLT, with benefits evident in as early as 30 days. Leqvio also reduced LDL-C by 59% over 360 days, outperforming placebo plus LLT by 35%. Data presented at ESC. Entresto
(sacubitril/ valsartan)Data from the Phase IV PARACHUTE-HF study in patients with heart failure with reduced ejection fraction due to chronic Chagas disease showed that Entresto outperformed enalapril on a composite endpoint of cardiovascular death, heart failure hospitalization or NT-proBNP change. Entresto was well tolerated, with no new safety signals identified. Data presented at ESC. Kesimpta
(ofatumumab)In the ARTIOS Phase IIIb study, patients with RMS who switched to Kesimpta after breakthrough disease on fingolimod or fumarate-based therapies showed a substantial reduction in disease activity. This was reflected in a low annualized relapse rate (ARR of 0.06 over 96 weeks), near-complete suppression of MRI activity, and over 90% of participants achieving no evidence of disease activity (NEDA-3). No new safety concerns were identified, regardless of prior disease-modifying treatment. In the separate ALITHIOS open-label extension study, more than 90% of naïve patients receiving Kesimpta showed no evidence of disease activity (NEDA-3) at 7 years, with no new safety concerns, reinforcing the benefit of introducing Kesimpta early. Data from both studies presented at ECTRIMS.
Selected transactions Novartis entered into an agreement to acquire Tourmaline Bio, a clinical-stage biopharmaceutical company developing pacibekitug, a Phase III-ready anti-IL-6 monoclonal antibody for atherosclerotic cardiovascular disease (ASCVD). In Phase II, pacibekitug reduced median high-sensitivity C-reactive protein (hsCRP) levels by up to 86% compared to placebo, with similar incidence rates of adverse events and serious adverse events. The transaction is expected to close on October 28, 2025. Novartis entered a second collaboration with Monte Rosa Therapeutics, in addition to the existing license agreement for VAV1 degraders, announced in October 2024. Under the new agreement, Novartis receives an exclusive license to an undisclosed discovery target and options to license two programs from Monte Rosa’s preclinical immunology portfolio.
Novartis continued its collaboration with Argo Biopharma, adding two new agreements: an exclusive license to an siRNA candidate currently in IND-enabling studies and expected to enter Phase I in 2026, and an option to exclusively license two second-generation siRNA molecules currently in development, with a right of first negotiation to the Phase II ANGPTL3 program.
Novartis entered into a global licensing and collaboration agreement with Arrowhead Pharmaceuticals for ARO-SNCA, a preclinical-stage siRNA therapy targeting alpha-synuclein for the treatment of synucleinopathies such as Parkinson’s disease. The agreement also includes additional collaboration targets leveraging Arrowhead’s proprietary Targeted RNAi Molecule (TRiM™) platform.
Capital structure and net debt
Retaining a good balance between investment in the business, a strong capital structure, and attractive shareholder returns remains a priority.
During the first nine months of 2025, Novartis repurchased a total of 66.4 million shares for USD 7.5 billion on the SIX Swiss Exchange second trading line. These repurchases included 49.1 million shares (USD 5.4 billion) under the USD 15 billion share buyback (announced in July 2023 and completed in July 2025) and 6.6 million shares (USD 0.8 billion) under the new up-to USD 10 billion share buyback announced in July 2025. In addition, 10.7 million shares (USD 1.3 billion) were repurchased to mitigate anticipated full-year dilution related to the equity-based compensation plans of associates. Further, 1.6 million shares (equity value of USD 0.2 billion) were repurchased from associates. In the same period, 11.7 million shares (equity value of USD 0.9 billion) were delivered to associates related to equity-based compensation plans. Consequently, the total number of shares outstanding decreased by 56.3 million versus December 31, 2024. These treasury share transactions resulted in an equity decrease of USD 6.8 billion and a net cash outflow of USD 7.7 billion.
Net debt increased to USD 20.4 billion at September 30, 2025, compared to USD 16.1 billion at December 31, 2024. The increase was mainly due to the free cash flow of USD 15.9 billion being more than offset by the USD 7.8 billion annual dividend payment, cash outflows for treasury share transactions of USD 7.7 billion and net cash outflow for M&A, intangible assets transactions and other acquisitions of USD 3.7 billion.
As of Q3 2025, the long-term credit rating for the company is Aa3 with Moody’s Ratings and AA- with S&P Global Ratings.
2025 outlook
Barring unforeseen events; growth vs. prior year in cc Net sales Expected to grow high single-digit Core operating income Expected to grow low-teens Foreign exchange impact
If late-October exchange rates prevail for the remainder of 2025, the foreign exchange impact for the year would be neutral to positive 1 percentage point on net sales and negative 2 percentage points on core operating income. The estimated impact of exchange rates on our results is provided monthly on our website.
Key figures1
Q3 2025 Q3 2024 % change 9M 2025 9M 2024 % change USD m USD m USD cc USD m USD m USD cc Net sales 13 909 12 823 8 7 41 196 37 164 11 11 Operating income 4 501 3 627 24 27 14 028 11 014 27 31 As a % of sales 32.4 28.3 34.1 29.6 Net income 3 930 3 185 23 25 11 563 9 119 27 29 EPS (USD) 2.04 1.58 29 31 5.94 4.50 32 35 Net cash flows from
operating activities6 571 6 286 5 16 880 13 426 26 Non-IFRS measures Free cash flow 6 217 5 965 4 15 941 12 618 26 Core operating income 5 460 5 145 6 7 16 960 14 635 16 18 As a % of sales 39.3 40.1 41.2 39.4 Core net income 4 330 4 133 5 6 13 522 11 822 14 17 Core EPS (USD) 2.25 2.06 9 10 6.94 5.83 19 21 1. Constant currencies (cc), core results and free cash flow are non-IFRS measures. An explanation of non-IFRS measures can be found on page 42 of the Condensed Interim Financial Report. Unless otherwise noted, all growth rates in this Release refer to same period in prior year.
Detailed financial results accompanying this press release are included in the Condensed Interim Financial Report at the link below:
https://ml-eu.globenewswire.com/resource/download/7781ab26-6902-4024-a9c8-49124629eb37/
Disclaimer
This press release contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995, that can generally be identified by words such as “anticipate,” “can,” “will,” “continue,” “ongoing,” “growth,” “launch,” “expect,” “expand,” “deliver,” “accelerate,” “guidance,” “outlook,” “priority,” “potential,” “momentum,” “commitment,” or similar expressions, or by express or implied discussions regarding potential new products, potential new indications for existing products, potential product launches, or regarding potential future revenues from any such products; or regarding results of ongoing clinical trials; or regarding potential future, pending or announced transactions; regarding potential future sales or earnings; or by discussions of strategy, plans, expectations or intentions, including discussions regarding our continued investment into new R&D capabilities and manufacturing; or regarding our capital structure. Such forward-looking statements are based on the current beliefs and expectations of management regarding future events and are subject to significant known and unknown risks and uncertainties. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those set forth in the forward-looking statements. You should not place undue reliance on these statements. There can be no guarantee that the investigational or approved products described in this press release will be submitted or approved for sale or for any additional indications or labeling in any market, or at any particular time. Nor can there be any guarantee that such products will be commercially successful in the future. Neither can there be any guarantee that the expected benefits or synergies from the transactions described in this press release will be achieved in the expected timeframe, or at all. In particular, our expectations could be affected by, among other things: uncertainties concerning global healthcare cost containment, including ongoing government, payer and general public pricing and reimbursement pressures and requirements for increased pricing transparency; uncertainties regarding the success of key products, commercial priorities and strategy; uncertainties in the research and development of new products, including clinical trial results and additional analysis of existing clinical data; our ability to obtain or maintain proprietary intellectual property protection, including the ultimate extent of the impact on Novartis of the loss of patent protection and exclusivity on key products; uncertainties regarding our ability to realize the strategic benefits, operational efficiencies or opportunities expected from our external business opportunities; uncertainties in the development or adoption of potentially transformational digital technologies, including artificial intelligence, and business models; uncertainties surrounding the implementation of our new IT projects and systems; uncertainties regarding potential significant breaches of information security or disruptions of our information technology systems; uncertainties regarding actual or potential legal proceedings, including regulatory actions or delays or government regulation related to the products and pipeline products described in this press release; safety, quality, data integrity, or manufacturing issues; our performance on and ability to comply with environmental, social and governance measures and requirements; major macroeconomic and geo- and socio-political developments, including the impact of any potential tariffs on our products or the impact of war in certain parts of the world; uncertainties regarding future global exchange rates; uncertainties regarding future demand for our products; and other risks and factors referred to in Novartis AG’s most recently filed Form 20-F and in subsequent reports filed with, or furnished to, the US Securities and Exchange Commission. Novartis is providing the information in this press release as of this date and does not undertake any obligation to update any forward-looking statements as a result of new information, future events or otherwise.All product names appearing in italics are trademarks owned by or licensed to Novartis.
About Novartis
Novartis is an innovative medicines company. Every day, we work to reimagine medicine to improve and extend people’s lives so that patients, healthcare professionals and societies are empowered in the face of serious disease. Our medicines reach nearly 300 million people worldwide.Reimagine medicine with us: Visit us at https://www.novartis.com and connect with us on LinkedIn, Facebook, X and Instagram.
Novartis will conduct a conference call with investors to discuss this news release today at 14:00 Central European time and 9:00 Eastern Time. A simultaneous webcast of the call for investors and other interested parties may be accessed by visiting the Novartis website. A replay will be available after the live webcast by visiting https://www.novartis.com/investors/event-calendar.
Detailed financial results accompanying this press release are included in the Condensed Interim Financial Report at the link below. Additional information is provided on our business and pipeline of selected compounds in late-stage development. A copy of today’s earnings call presentation can be found at https://www.novartis.com/investors/event-calendar.
Important dates October 30, 2025 Immunology pipeline event at ACR (virtual) November 19-20, 2025 Meet Novartis Management 2025 (London, UK) December 1, 2025 Social Impact & Sustainability annual investor event (virtual) February 4, 2026 Fourth quarter & full year 2025 results # # #
Please find full media release in English attached and on the following link:
Media Release (PDF)Further language versions are available through the following links:
German version is available through the following link:
Medienmitteilung (PDF)
Continue Reading
- Q3 net sales grew +7% (cc1, +8% USD) and core operating income1 grew +7% (cc, +6% USD)
-
‘I called my parents after two weeks and said I’m not coming back’ – The Irish Times
Two-and-a-half years ago, Léna Descottes (29) thought her time in Ireland was over. Despite being a traditional-music performer who had played in venues across the country, she had spent the previous six months sleeping primarily in her car….
Continue Reading
