In the most highly anticipated opening of the season, the Fondation Cartier for Contemporary Art will unveil its new Parisian address – and it’s not…
Blog
-

China and Iran shine as Pakistan falters in Asia-Pacific Goalball championship
The Asia-Pacific Goalball Championship 2025, Saturday, witnessed an exciting day of competition, with China and Iran registering strong victories, while Pakistan faced defeat in its group fixture.
In the men’s category, Saudi Arabia defeated…
Continue Reading
-

S Africa storm past S Lanka in rain-hit WCup encounter
COLOMBO: South Africa’s juggernaut rolled on in the Women’s World Cup as they crushed Sri Lanka by 10 wickets in a rain-marred contest in Colombo on Friday. In a game reduced to 20 overs, the Proteas first tied down Sri Lanka to 105-7 and…
Continue Reading
-

PM Shehbaz Sharif hails Pakistan’s economic recovery and diplomatic achievements
Prime Minister Shehbaz Sharif has lauded Pakistan’s progress toward economic stability and diplomatic success, attributing it to effective governance, hard work, and a shared national vision.
Speaking to the media in Jahanian, Punjab, the…
Continue Reading
-

Palestinians, Israel disagree on whether Gaza’s crucial Rafah crossing will reopen Monday
LONDON: Egypt is expected to take the lead in an international stabilization force being developed to oversee security inside Gaza under a proposed UN Security Council mandate backed by the US and European partners,…Continue Reading
-

Know where to watch ARG vs MOR football live streaming in India
Six-time champions Argentina will take on Morocco in the FIFA U-20 World Cup 2025 final at Chile’s Estadio Nacional Julio Martínez Prádanos in the early hours of Monday morning.
The Argentina vs Morocco FIFA U-20 World Cup 2025 final will be…
Continue Reading
-

Unlikely Hero Scores Stoppage Time Winner
Ronald Araújo scored a 93rd-minute winner for Barcelona in their La Liga clash with Catalan rivals Girona on Saturday as Hansi Flick’s side moved to the top of the table.
La Blaugrana raced out of the blocks as they sought redemption from…
Continue Reading
-

The Prem: Leicester Tigers 22-20 Bath
In the clubs’ first meeting since Bath won a closely fought Twickenham showpiece in June, it was the away side who struck first.
Santi Carreras, in his first Bath start, kicked forward in midfield for his team-mates to chase. The ball was spilled…
Continue Reading
-

T-DXd vs T-DM1 in HER2+ Early BC
DESTINY-Breast05 (NCT04622319), presented by Dr. Charles E. Geyer (Pittsburgh, United States of America) at the ESMO Congress 2025, is a pivotal phase 3, open-label, randomized trial evaluating trastuzumab deruxtecan (T-DXd) versus the standard-of-care trastuzumab emtansine (T-DM1) in patients with HER2-positive early breast cancer (eBC) who had residual invasive disease after neoadjuvant therapy. The study was designed to determine whether T-DXd could improve long-term outcomes for this high-risk population compared with T-DM1, the established post-neoadjuvant standard of care
Background
Patients with HER2-positive early breast cancer who have residual invasive disease following neoadjuvant chemotherapy and anti-HER2 therapy face a high risk of recurrence, particularly distant relapse. T-DM1 became the standard post-neoadjuvant treatment following the KATHERINE trial, but outcomes remain suboptimal for patients with high residual disease burden. Trastuzumab deruxtecan (T-DXd), a next-generation HER2-directed antibody–drug conjugate with a potent topoisomerase I inhibitor payload, has shown marked efficacy in metastatic settings, prompting investigation into its use in the early disease setting to reduce recurrence risk.
Methods
In DESTINY-Breast05, 1,635 patients with HER2-positive eBC and residual invasive disease after neoadjuvant taxane-based chemotherapy and HER2-targeted therapy were randomized 1:1 to receive either:
- T-DXd (5.4 mg/kg) every 3 weeks,for a total of 14 cycles.
- T-DM1 (3.6 mg/kg) every 3 weeks,for a total of 14 cycles.
Eligible patients were considered high risk for recurrence, defined by clinical stages T4, N0–3, M0 or T1–3, N2–3, M0 at presentation, or residual nodal disease after neoadjuvant therapy.
The primary endpoint was invasive disease-free survival (IDFS), with disease-free survival (DFS) as a key secondary endpoint. Additional endpoints included overall survival (OS), distant recurrence-free interval, brain metastasis–free interval (BMFI), and safety.
Results
At the data cutoff of July 2, 2025, median follow-up was 29.9 months in the T-DXd arm and 29.7 months in the T-DM1 arm.
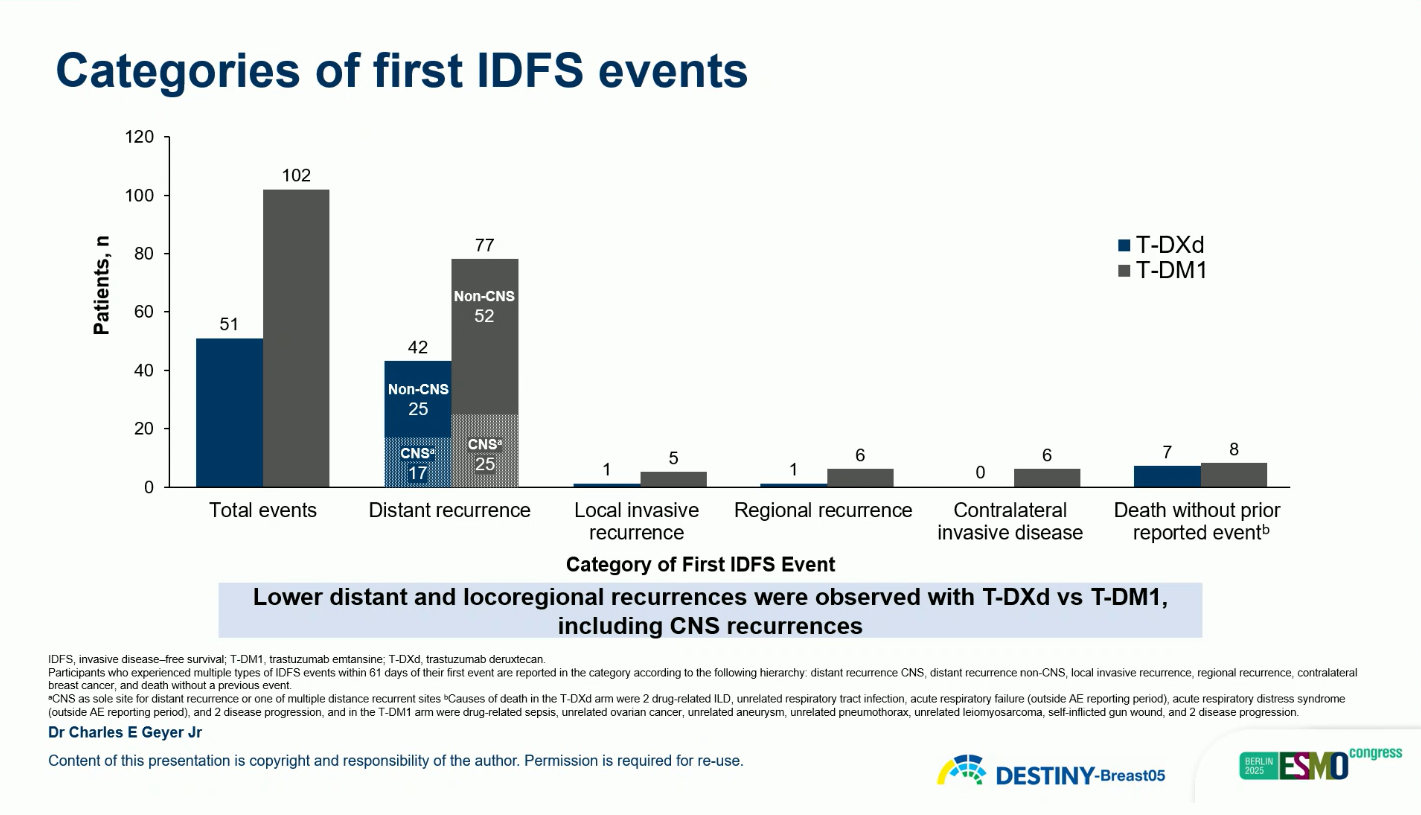
- IDFS events: 6.2% with T-DXd vs 12.5% with T-DM1
- DFS events: 6.4% vs 12.6%
- Hazard ratio (HR): 0.47 for both IDFS and DFS (95% CI: 0.34–0.66; p < 0.0001)
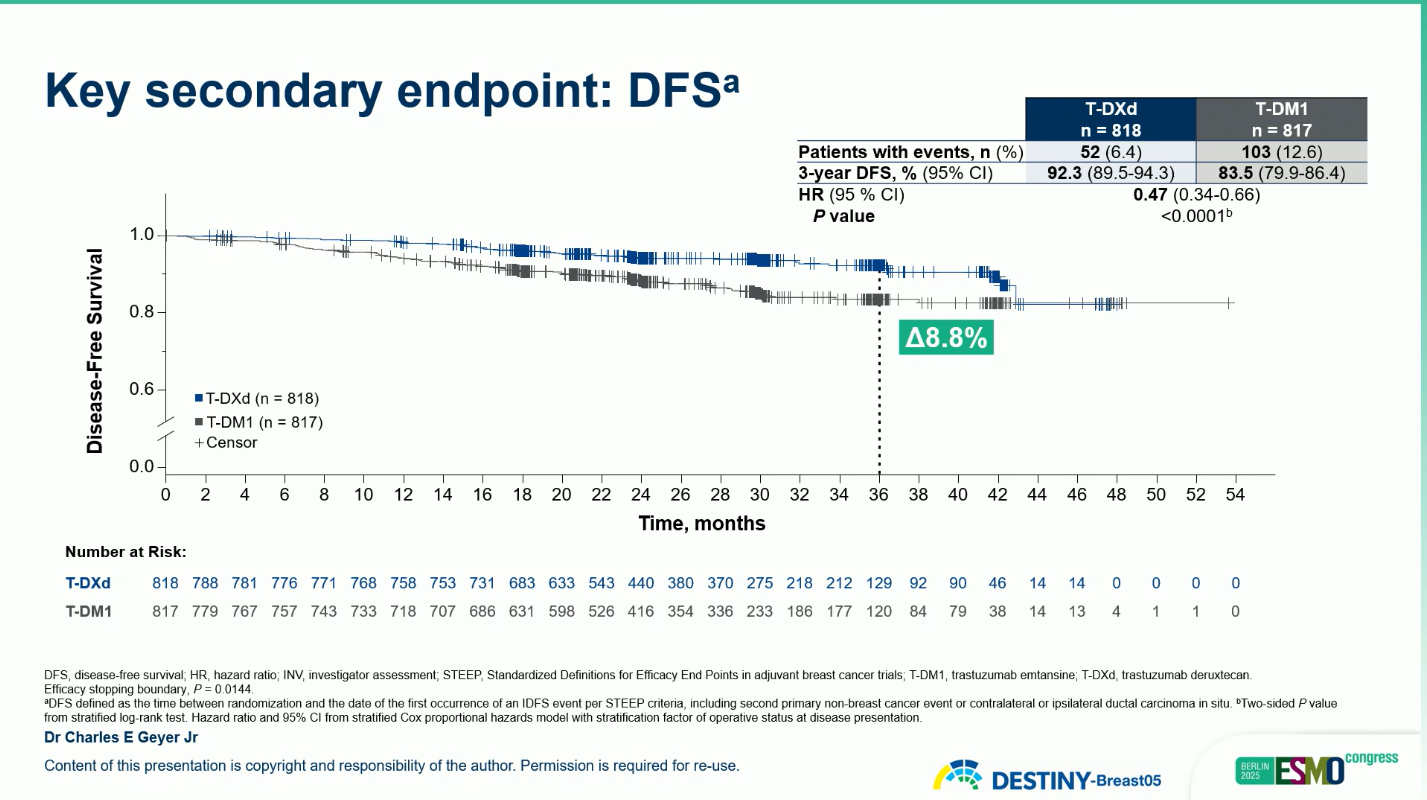
Similarly, the key secondary endpoint of disease-free survival (DFS) demonstrated a parallel benefit, with a hazard ratio of 0.47 (95% CI, 0.34–0.66; p < 0.0001). The 3-year DFS rate was 92.3% with T-DXd compared with 83.5% with T-DM1, corresponding to an 8.8% absolute gain.
These findings represent a 53% reduction in risk of invasive disease recurrence or death with T-DXd compared with T-DM1.
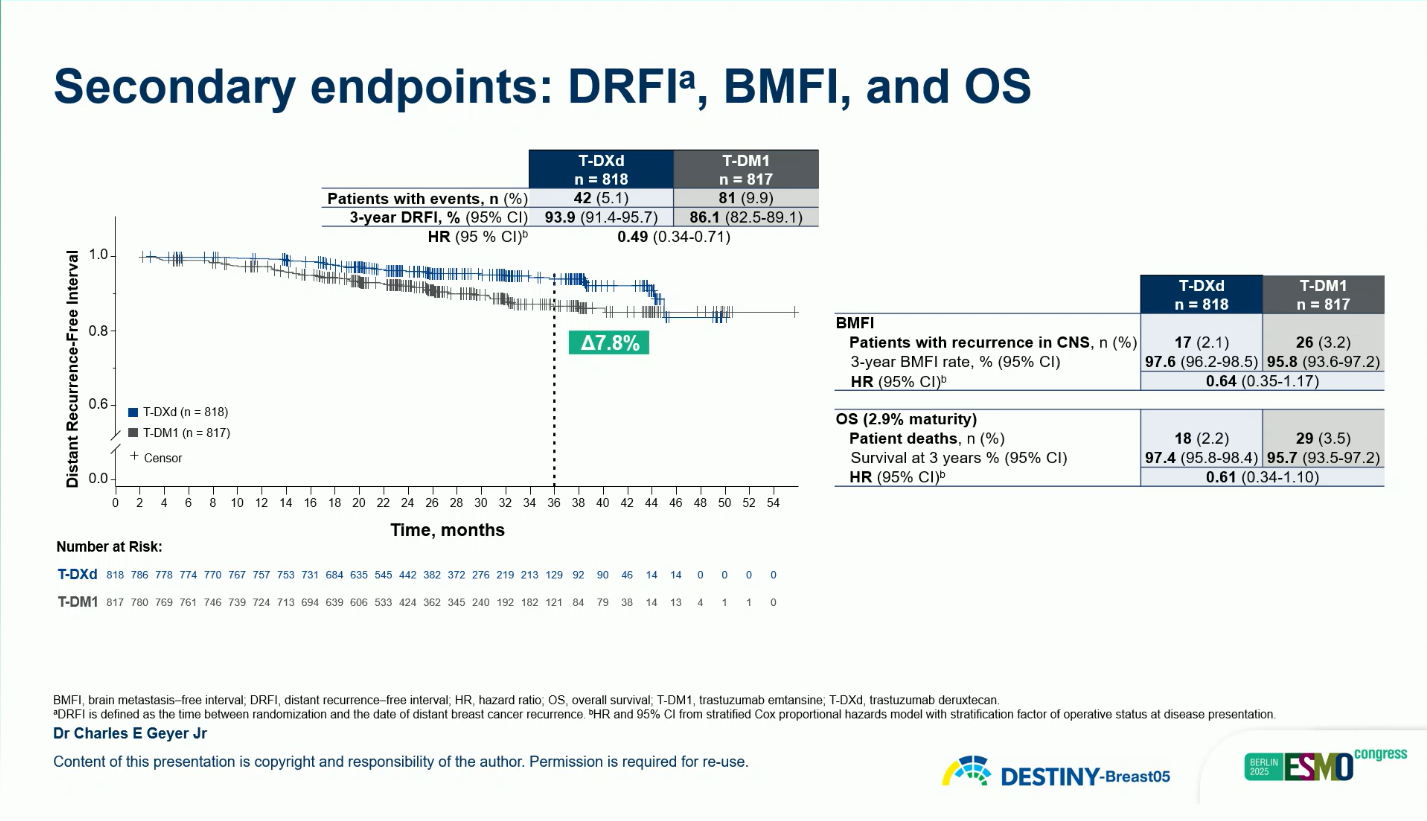
A clinically meaningful improvement in BMFI was also observed (HR 0.64; 95% CI 0.35–1.17), suggesting enhanced control of central nervous system relapse.
Safety
The overall safety profile of T-DXd was manageable and consistent with prior studies.
- Grade ≥3 TEAEs: 50.6% (T-DXd) vs 51.9% (T-DM1)
- Adjudicated interstitial lung disease (ILD): 9.6% (2 grade 5 cases) vs 1.6% (none grade 5)
- Treatment-related deaths: 0.4% (T-DXd) vs 0.6% (T-DM1)
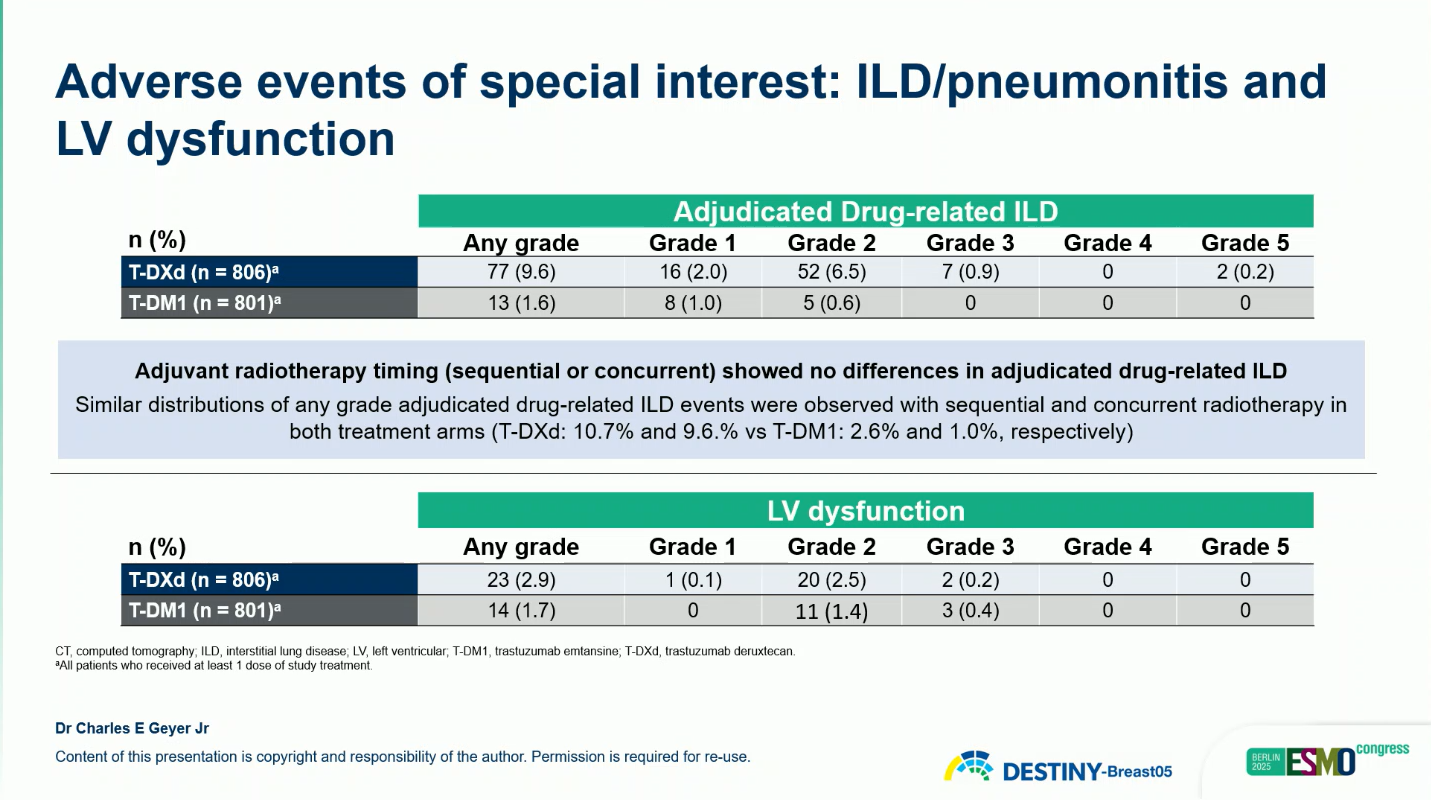
Most ILD events were grade 1–2 and resolved with treatment modification or corticosteroids. No new safety signals were identified.
Conclusions
The DESTINY-Breast05 trial demonstrated that trastuzumab deruxtecan (T-DXd) offers a statistically significant and clinically meaningful improvement in both invasive disease-free survival (IDFS) and disease-free survival (DFS) compared with trastuzumab emtansine (T-DM1) in patients with HER2-positive early breast cancer who had residual invasive disease following neoadjuvant therapy.
These findings mark a pivotal advance in the post-neoadjuvant management of HER2-positive breast cancer. By extending the proven efficacy of T-DXd beyond the metastatic setting into early-stage, high-risk disease, the results highlight its potential to redefine the standard of care for patients who previously had limited options after incomplete response to neoadjuvant therapy. Importantly, the benefit was consistent across all major subgroups, including hormone receptor–positive and –negative disease, as well as across regions and baseline disease characteristics, underscoring the robustness of the findings.
You can read the full abstract here.
Continue Reading
-
Just a moment…
Just a moment… This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
Continue Reading
