- Sinner, Fritz, Shelton headline Vienna & Basel fields in potentially crucial week in Live Race ATP Tour
- Sinner in line for Bublik rematch in Vienna, Musetti & Tsitsipas in R1 showdown ATP Tour
- Sinner vs. Altmaier Prediction at the Erste Bank Open…
Blog
-
Sinner, Fritz, Shelton headline Vienna & Basel fields in potentially crucial week in Live Race – ATP Tour
-
Final Analysis of COMPASSION-15 Presented at ESMO 2025
HONG KONG, Oct. 19, 2025 /PRNewswire/ — On October 19, 2025, Akeso (9926.HK) announced the final analysis results from the COMPASSION-15/AK104-302 study at the 2025 European Society of Medical Oncology Congress (ESMO 2025) . COMPASSION-15 is a Phase III clinical trial evaluating cadonilimab, Akeso’s first-in-class PD-1/CTLA-4 bispecific antibody, in combination with oxaliplatin and capecitabine as first-line treatment for unresectable, locally advanced, recurrent, or metastatic gastric or gastroesophageal junction (G/GEJ) adenocarcinoma. Professor Shen Lin, principal investigator from Peking University Cancer Hospital, presented the findings in an oral session at ESMO 2025.
In this final analysis presented at ESMO 2025 (median follow-up: 33.9 months), the cadonilimab regimen demonstrated enhanced long-term survival benefits in first-line advanced G/GEJ adenocarcinoma. With the extended follow-up period and more mature data, the cadonilimab treatment regimen showed a further reduction in the risk of death compared to the control group. This consistent benefit was observed across all PD-L1 expression subgroups.
The interim analysis of COMPASSION-15, with a median follow-up of 18.7 months, was previously published in Nature Medicine in January 2025. The data presented at ESMO 2025 were analyzed using the same statistical methodology.
COMPASSION-15 2025 ESMO Data
In the intent-to-treat (ITT) population:
- With long-term follow-up, the cadonilimab regimen demonstrated a significant 39% reduction in the risk of death (OS HR 0.61) versus the control group, showing further improvement over the data from the median 18.7-month follow-up (OS HR 0.66).
- With extended follow-up, in the PD-L1 CPS ≥5 population, the cadonilimab regimen demonstrated a significant 51% reduction in the risk of death (OS HR 0.49; p < 0.001) compared to the control group, showing further improvement over the data from the median 18.7-month follow-up (OS HR 0.58).
- With long-term follow-up, in the PD-L1 CPS <5 population, the cadonilimab regimen showed a significant 24% reduction in the risk of death (OS HR 0.76; 95% CI: 0.59-0.99; p = 0.019) versus the control group, with a strengthening trend of benefit compared to the data from the median 18.7-month follow-up (OS HR 0.75; 95% CI: 0.56-1.00).
- Following extended the follow-up period, the cadonilimab combination regimen maintained a favorable safety profile, with no new safety signals emerging.
In the COMPASSION-15 study, patients with PD-L1 CPS <5 (low expression) and CPS <1 (negative expression) are 49.8% and 23%, respectively, of the ITT population. This represents a higher proportion of PD-L1 low and negative patient population in COMPASSION-15 compared to previous Phase III trials of other immune checkpoint inhibitors used in the treatment of first-line gastric cancer. Previous studies have shown limited responses to PD-1/L1 inhibitors in PD-L1 low-expression or negative patients.
Cadonilimab was approved by the NMPA in September 2024 for the first-line treatment for advanced gastric cancer, offering a new and effective immunotherapy option. Cadonilimab has been included in the 2025 CSCO Gastric Cancer Guidelines as the only Category I recommendation (Level 1A evidence) for first-line immunotherapy, regardless of PD-L1 expression, and is currently widely used in clinical practice.
Forward-Looking Statement of Akeso, Inc.
This announcement by Akeso, Inc. (9926.HK, “Akeso”) contains “forward-looking statements”. These statements reflect the current beliefs and expectations of Akeso’s management and are subject to significant risks and uncertainties. These statements are not intended to form the basis of any investment decision or any decision to purchase securities of Akeso. There can be no assurance that the drug candidate(s) indicated in this announcement or Akeso’s other pipeline candidates will obtain the required regulatory approvals or achieve commercial success. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements.Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of pharmaceutical industry regulation and health care legislation in P.R.China, the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; Akeso’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of the Akeso’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions.
Akeso does not undertake any obligation to publicly revise these forward-looking statements to reflect events or circumstances after the date hereof, except as required by law.
About Akeso
Akeso (HKEX: 9926.HK) is a leading biopharmaceutical company committed to the research, development, manufacturing and commercialization of the world’s first or best-in-class innovative biological medicines. Founded in 2012, the company has created a unique integrated R&D innovation system with the comprehensive end-to-end drug development platform (ACE Platform) and bi-specific antibody drug development technology (Tetrabody) as the core, a GMP-compliant manufacturing system and a commercialization system with an advanced operation mode, and has gradually developed into a globally competitive biopharmaceutical company focused on innovative solutions. With fully integrated multi-functional platform, Akeso is internally working on a robust pipeline of over 50 innovative assets in the fields of cancer, autoimmune disease, inflammation, metabolic disease and other major diseases. Among them, 24 candidates have entered clinical trials (including 15 bispecific/multispecific antibodies and bispecific ADCs. Additionally, 7 new drugs are commercially available. Through efficient and breakthrough R&D innovation, Akeso always integrates superior global resources, develops the first-in-class and best-in-class new drugs, provides affordable therapeutic antibodies for patients worldwide, and continuously creates more commercial and social values to become a global leading biopharmaceutical enterprise.For more information, please visit https://www.akesobio.com/en/about-us/corporate-profile/ and follow us on Linkedin.
SOURCE Akeso, Inc.

Continue Reading
-

Bangladesh garment exporters fear $1bn losses after huge airport fire | Business and Economy News
The fire gutted import cargo terminals areas at Dhaka airport, destroying an estimated $1bn of ‘urgent air shipments’.
Published On 19 Oct 2025
A fire that decimated a cargo complex in…
Continue Reading
-
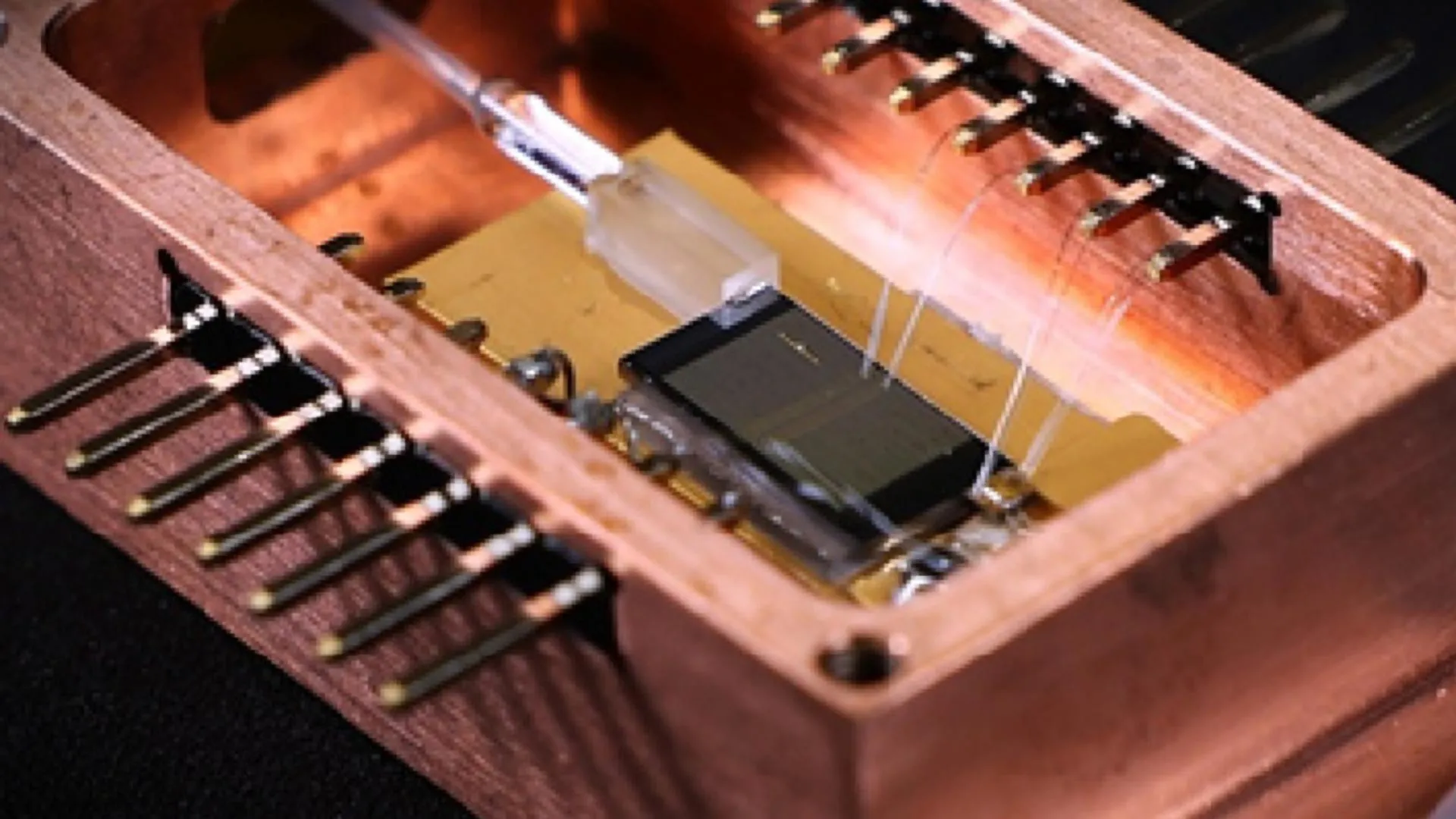
This tiny laser could transform how we see and sense the world
Laser technology plays a vital role in modern life, supporting everything from precise scientific measurements to advanced communication systems. It underpins technologies such as self-driving vehicles, high-speed fiber optic networks, and even…
Continue Reading
-

Former Big Brother contestant sues for disability after washing dishes on set
Former Big Brother contestant Shilo Shalom has filed a lawsuit against the National Insurance Institute, demanding recognition of what he claims is a lasting disability stemming from an injury he sustained while washing dishes during the 2024…
Continue Reading
-

‘Battlefield 6’ Season 1 release date, content, updates, and more
Battlefield 6 has had a stellar first week, becoming the most successful game in the franchise and EA has no plans to stop supporting the game as new content is planned before the end of the month.
- READ MORE: ‘Battlefield 6’ review: modern…
Continue Reading
-

Abadia Fall 2025 at Riyadh Fashion Week – WWD
- Abadia Fall 2025 at Riyadh Fashion Week WWD
- Vivienne Westwood Show Opens Riyadh Fashion Week, as Saudis Highlight Creative Side The New York Times
- Saudi labels ‘honor roots’ at Riyadh Fashion Week Arab News
- Mushroom skin bag and Riyadh…
Continue Reading
-
![Leem Fall 2025 at Riyadh Fashion Week [PHOTOS] – WWD](https://afnnews.qaasid.com/wp-content/uploads/2025/10/leem-f25-riyadh-08.jpg)
Leem Fall 2025 at Riyadh Fashion Week [PHOTOS] – WWD
- Leem Fall 2025 at Riyadh Fashion Week [PHOTOS] WWD
- Vivienne Westwood Show Opens Riyadh Fashion Week, as Saudis Highlight Creative Side The New York Times
- Saudi labels ‘honor roots’ at Riyadh Fashion Week Arab News
- Mushroom skin bag and Riyadh…
Continue Reading
-

Your cheese may contain thousands of microplastic particles
Milk has around 350 particles per kilogram, while cheese contains at least 1,000 per kilogram. PET, polyethene and polypropylene are the common plastics, which are generally found in packaging, which could be the reason behind contamination. But…
Continue Reading
-

212Pb-DOTAMTATE Shows Impressive Antitumor Activity in Previously Treated GEP-NETS | Targeted Oncology
212Pb-DOTAMTATE (AlphaMedix) showed clinically meaningful antitumor activity with sustained and durable responses along with a manageable safety profile in patients with peptide receptor radionuclide therapy (PRRT)-exposed, somatostatin receptor (SSTR)-positive, unresectable or metastatic gastroenteropancreatic neuroendocrine tumors (GEP-NETs), according to results from the ALPHAMEDIX-02 trial (NCT05153772).1
Per investigator review, the objective response rate (ORR) was 34.6% (9/26 patients), consisting of all partial responses. None of the responders had progressed at the time of the data cutoff. Sixteen patients (61.5%) had stable disease for a disease control rate of 96.2% (95% CI, 80.4%-99.9%). There was 1 patient with progressive disease. The ORR per blinded independent central review was 19.2% and the disease control rate was 100%.
The investigator-assessed 18-month progression-free survival (PFS) and overall survival (OS) rates were 82.6% (59.0-93.3) and 85.1% (58.5%-95.2%), respectively. The PFS per BICR was 88.0% (95% CI, 67.3%-96.0%).
212Pb-DOTAMTATE was determined by investigators to have an acceptable side effect profile, with the majority of treatment-emergent adverse events (TEAEs) being grade 1 or 2.
“The pivotal phase 2 ALPHAMEDIX-02 study presents a favorable benefit/risk profile of 212Pb-based targeted alpha therapy in radioligand therapy (RLT)-exposed patients with advanced GEP-NETS where few effective treatment options are available,” said lead study author Jonathan Strosberg, MD,a professor and leader of the Neuroendocrine Tumor Division and the Department of Gastrointestinal Oncology Research Program at Moffitt Cancer Center.
Study Design and Rationale of ALPHAMEDIX-02
“Effective treatment options are limited for patients with GEP-NETs whose disease progresses after PRRT with beta-emitting 177Lu-labelled somatostatin analogs (SSA),” said Strosberg.
Accordingly, Strosberg et al explored the next-generation SSTR-targeted RLT 212Pb-DOTAMTATE in this patient population. 212Pb-DOTAMTATE “utilizes the high-energy and shorter-range alpha emission, which leads to highly effective dsDNA breakage and may result in more targeted oncologic effects,” explained Strosberg.
Overall, the open-label, multicenter, phase 2 ALPHAMEDIX-02 study explored 212Pb-DOTAMTATE in patients with both PRRT-naïve (cohort 1) and PRRT-exposed (cohort 2) metastatic GEP-NETs that was histologically confirmed, had positive SSA imaging, and had at least 1 site of measurable disease.
Previously reported data for cohort 1 showed that 212Pb-DOTAMTATE achieved an ORR of 54.3% and a manageable safety profile in PRRT-naïve patients. Results for PRRT-exposed patients (cohort 2) were shared by Strosberg at ESMO.
Cohort 2 comprised 26 PRRT-exposed patients who had progressive disease following treated with up to 4 doses of 177Lu-SSA. Patients had received their last 177Lu-SSA dose at least 6 months before the trial. All patients received 212Pb-DOTAMTATE at 67.6 μCi/kg every 8 weeks for up to 4 cycles. The primary end point was ORR (RECIST1.1) and safety. Secondary outcome measures included progression free survival and overall survival.
What Was the Safety Profile of 212Pb-DOTAMTATE in AMEDIX-02
“212Pb-DOTAMTATE demonstrated an acceptable side effect profile, where most (57.7%) treatment-emergent adverse events (TEAEs) were grade 1 or 2, allowing the majority (84.6%) of patients to complete all 4 doses and 0 TEAEs leading to treatment discontinuation,” said Strosberg.
The most common grade 1/2 TEAEs were alopecia (84.6%), fatigue (76.9%), nausea (69.2%), diarrhea (38.5%), dysphagia (34.6%), vomiting (30.8%), decreased appetite (30.8%), abdominal pain (26.9%), and lymphopenia (23.1%).
Eleven patients (42.3%) experienced grade ≥3 TEAEs. These included lymphopenia (n = 4), dysphagia (n = 3), vomiting (n = 2), deceased appetite (n = 1), and fatigue (n = 1). Five patients had treatment-emergent serious adverse events and there were no TEAE-related patient deaths.
Specifically focusing on dysphagia, Strosberg explained that the condition emerged as a chronic toxicity which was “kind of a surprising side effect––it’s not something that we anticipated when we started this trial.” He added, “Manometry may demonstrate esophageal dysmotility in patients with dysphagia. Botox injection to the lower esophageal sphincter provides relief in many cases and some patients have required minimally-invasive surgery.”
What Were Patient Characteristics in AMEDIX-02?
The mean patient age was 61.8 years and the median time since diagnosis was 7.2 years (range, 2.8-18.8). Over three-fourths of patients had grade 1 or 2 disease. The primary tumor sites were the pancreas and small intestine at 11 patients each. All patients had prior RLT, 96.2% had prior SSAs, and 88.5% had surgical resection prior to enrollment. Further, 15 patients had received chemotherapy, 15 had received TKIs, 7 had embolization, and 5 had received EBRT.
What Are the Next Steps for 212Pb-DOTAMTATE?
In a press release reporting these results, Christopher Corsico, MD, Global Head of Development at Sanofi, which codevelops 212Pb-DOTAMTATE with Orano Med, stated, “The promising ALPHAMEDIX-02 results represent a significant step forward, reinforcing the potential of targeted alpha therapy to deliver precise treatment for GEP-NETs.”2
Corsico added, “These data, demonstrating clinically meaningful activity and a manageable safety profile, underscore our unrelenting commitment to developing innovative therapies for patients with difficult-to-treat cancers. We look forward to advancing [212Pb-DOTAMTATE] and working with Orano Med and regulators to bring this important treatment to the GEP-NET community as soon as possible.”
References
- Strosberg JR, Naqvi S, Cohn A, et al. Efficacy and safety of targeted alpha therapy 212Pb-DOTAMTATE in patients with unresectable or metastatic gastroenteropancreatic neuroendocrine tumors (GEP-NETs) previously treated with peptide receptor radionuclide therapy (PRRT): Results of a phase II study. Presented at: 2025 ESMO Congress; October 17-21, 2025; Berlin, Germany. Abstract 1032O.
- AlphaMedixTM (212Pb-DOTAMTATE) achieved all primary efficacy endpoints in phase 2 study, demonstrating clinically meaningful benefits in patients with gastroenteropancreatic neuroendocrine tumors. Posted online October 8, 2025. Accessed October 19, 2025. https://www.sanofi.com/en/media-room/press-releases/2025/2025-10-08-05-00-00-3163053
Continue Reading