Astronomers have discovered a 2,300-foot-wide (700 meters) asteroid hidden in the sun’s glare, and it’s whizzing through our solar system at a near record-breaking pace.
The skyscraper-size asteroid, named 2025 SC79, loops around the sun once…

Astronomers have discovered a 2,300-foot-wide (700 meters) asteroid hidden in the sun’s glare, and it’s whizzing through our solar system at a near record-breaking pace.
The skyscraper-size asteroid, named 2025 SC79, loops around the sun once…

Caltech researchers co-lead new study refining what we know about the ghostly particles
Very early on in our universe, when it was a seething hot cauldron of energy, particles made of matter and antimatter bubbled into existence in equal…

LOS ANGELES, Oct. 22, 2025 /PRNewswire/ — Armata Pharmaceuticals, Inc. (NYSE American: ARMP) (“Armata” or the “Company”), a clinical-stage biotechnology company focused on the development of high-purity, pathogen-specific bacteriophage therapeutics for the treatment of antibiotic-resistant and difficult-to-treat bacterial infections, today highlighted positive results from its recently completed Phase 2a diSArm study of AP-SA02 as a potential treatment for complicated Staphylococcus aureus (“S. aureus“) bacteremia (“SAB”) in a late-breaking oral presentation at IDWeek 2025™.
The abstract, titled, “A Phase 2a Randomized, Double-Blind, Controlled Trial of the Efficacy and Safety of an Intravenous (IV) Bacteriophage Cocktail (AP-SA02) vs. Placebo in Combination with Best Available Antibiotic Therapy (BAT) in Patients with Complicated Staphylococcus aureus Bacteremia,” was accepted as a highly coveted late-breaking abstract for oral presentation, and was presented by Dr. Loren G. Miller, M.D., M.P.H., Professor of Medicine, David Geffen School of Medicine at UCLA, Chief, Division of Infectious Diseases at Harbor-UCLA Medical Center and the Lundquist Institute.
“The results of the diSArm study confirm, for the first time in a randomized clinical trial, the efficacy of intravenous phage therapy for S. aureus bacteremia, and we are very pleased to highlight these compelling data in an oral presentation at IDWeek,” stated Dr. Miller. “The results of this rigorously designed study provide strong rationale for advancement into a Phase 3 superiority study that, if successful, would support its use in clinical practice for Staphylococcus aureus bacteremia. High-purity, phage-based therapeutics like AP-SA02 have the potential to become the new standard of care for this common, extremely severe, and often deadly infection.”
“The positive results from the diSArm study represent another significant achievement for Armata as we aim to advance AP-SA02 into a pivotal trial,” stated Dr. Deborah Birx, Chief Executive Officer of Armata. “I would like to thank Dr. Miller and the other investigators who contributed to the efficient execution of the diSArm study, and I look forward to working with many of them on a proposed pivotal study next year. I would also like to thank Dr. Vance Fowler who served as the chair of the independent blinded adjudication committee that independently confirmed safety and efficacy findings throughout the Phase 2 trial. Finally, I would like to express my gratitude to the patients who participated in this important study, and acknowledge our partners at the U.S. Department of Defense, and our significant shareholder, Innoviva, who have each provided critical support to make this early breakthrough possible.”
Data highlights:
The Phase 2a study enrolled and dosed 42 patients, with 29 randomized to AP-SA02 in addition to BAT and 13 to placebo (BAT alone). Methicillin-resistant S. aureus (“MRSA”) was the causative pathogen in ~38% of both the AP-SA02 and placebo groups.
Clinical response was assessed in the intent-to-treat (ITT) population at Test of Cure (“TOC”) on day 12, one week post-BAT, and End of Study (“EOS”) four weeks after BAT completion. Safety analysis also included data from the Phase 1b portion of the trial (n=8).
Day 12 clinical response rates were higher in the AP-SA02 group — 88% (21/24) versus 58% (7/12) in the placebo group as assessed by blinded site investigators (“PI”) (p = 0.047), and 83% (20/24) in the AP-SA02 group versus 58% (7/12) in the placebo group as assessed by the blinded Adjudication Committee (“AC”).
Non-response/relapse rates were evaluated at the two later timepoints — one week post-BAT and EOS. No patients in the AP-SA02 group experienced non-response or relapse (0%) by either PI or AC assessment. In contrast, the placebo group showed 25% non-response/relapse at both timepoints reported by the PI (p = 0.017) and 22% non-response/relapse at one week post-BAT (p = 0.025) and 25% at EOS (p = 0.02) by the AC.
Patients treated with AP-SA02 showed trends toward rapid normalization of C-reactive protein, shorter time to negative blood culture, quicker time to resolution of signs and symptoms at the infection site, shorter intensive care unit and hospital utilization.
AP-SA02 was well-tolerated with no serious adverse events related to the study drug. Treatment-emergent adverse events occurred in 6% (2/35) and 0% (0/15) in the AP-SA02 and placebo groups, respectively: one patient with transient liver enzyme elevation and one patient with hypersensitivity that resolved with discontinuation of vancomycin.
New findings demonstrate that the defined and reproducible genomic variants present in AP-SA02 Drug Product may provide an immediate advantage, enabling rapid, strain-specific response to each patient’s S. aureus isolate. These characterized variants can expand from as little as 2% to dominance when infecting certain patient isolates in vitro, highlighting that these variants are favored for their enhanced ability to infect those strains and the importance of integrating this diversity into Armata’s phage cocktail from the outset. This inherent flexibility may be central to achieving optimal therapeutic efficacy.
Conclusions:
About IDWeek 2025™
IDWeek 2025™ is a joint annual meeting of the Infectious Diseases Society of America (IDSA), the Society for Healthcare Epidemiology of America (SHEA), the HIV Medicine Association (HIVMA), the Pediatric Infectious Diseases Society (PIDS) and the Society of Infectious Diseases Pharmacists (SIDP). With the theme “Advancing Science, Improving Care,” IDWeek features the latest science and bench-to-bedside approaches in prevention, diagnosis, treatment and epidemiology of infectious diseases, including HIV, across the lifespan. IDWeek 2025™ takes place October 19-22 in Atlanta, GA. For more information, visit www.idweek.org.
About AP-SA02 and diSArm Study
Armata is developing AP-SA02, a fixed multi-phage phage cocktail, for the treatment of complicated bacteremia caused by Staphylococcus aureus (S. aureus), including methicillin-sensitive S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) strains.
The diSArm study (NCT05184764) was a Phase 1b/2a, multicenter, randomized, double-blind, placebo-controlled, multiple ascending dose escalation study of the safety, tolerability, and efficacy of intravenous AP-SA02 in addition to best available antibiotic therapy (BAT) compared to BAT alone (placebo) for the treatment of adults with complicated S. aureus bacteremia. The results from the diSArm study are an important step forward in Armata’s effort to confirm the potent antimicrobial activity of phage therapy and the completion of the study represents a significant milestone in the development of AP-SA02, moving Armata one step closer to introducing an effective new treatment option to patients suffering from complicated S. aureus bacteremia.
The Phase 1b/2a clinical development of AP-SA02 was partially supported by a $26.2 million Department of Defense (DoD) award, received through the Medical Technology Enterprise Consortium (MTEC) and managed by the Naval Medical Research Command (NMRC) – Naval Advanced Medical Development (NAMD) with funding from the Defense Health Agency and Joint Warfighter Medical Research Program.
About Armata Pharmaceuticals, Inc.
Armata is a clinical-stage biotechnology company focused on the development of high-purity pathogen-specific bacteriophage therapeutics for the treatment of antibiotic-resistant and difficult-to-treat bacterial infections using its proprietary bacteriophage-based technology. Armata is developing and advancing a broad pipeline of natural and synthetic phage candidates, including clinical candidates for Pseudomonas aeruginosa, Staphylococcus aureus, and other important pathogens. Armata is committed to advancing phage therapy with drug development expertise that spans bench to clinic including in-house phage-specific current Good Manufacturing Practices (“cGMP”) manufacturing to support full commercialization.
Forward Looking Statements
This communication contains “forward-looking” statements as defined by the Private Securities Litigation Reform Act of 1995. These statements relate to future events, results or to Armata’s future financial performance and involve known and unknown risks, uncertainties and other factors which may cause Armata’s actual results, performance or events to be materially different from any future results, performance or events expressed or implied by the forward-looking statements. In some cases, you can identify these statements by terms such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” or the negative of those terms, and similar expressions. These forward-looking statements reflect management’s beliefs and views with respect to future events and are based on estimates and assumptions as of the date of this communication and are subject to risks and uncertainties including risks related to Armata’s development of bacteriophage-based therapies; ability to staff and maintain its production facilities under fully compliant cGMP; ability to meet anticipated milestones in the development and testing of the relevant product; ability to be a leader in the development of phage-based therapeutics; ability to achieve its vision, including improvements through engineering and success of clinical trials; ability to successfully complete preclinical and clinical development of, and obtain regulatory approval of its product candidates and commercialize any approved products on its expected timeframes or at all; and Armata’s estimates regarding anticipated operating losses, capital requirements and needs for additional funds. Additional risks and uncertainties relating to Armata and its business can be found under the caption “Risk Factors” and elsewhere in Armata’s filings and reports with the U.S. Securities and Exchange Commission (the “SEC”), including in Armata’s Annual Report on Form 10-K, filed with the SEC on March 21, 2025, and in its subsequent filings with the SEC.
Armata expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in Armata’s expectations with regard thereto or any change in events, conditions or circumstances on which any such statements are based.
Media Contacts:
At Armata:
Pierre Kyme
[email protected]
310-665-2928
Investor Relations:
Joyce Allaire
LifeSci Advisors, LLC
[email protected]
212-915-2569
SOURCE Armata Pharmaceuticals, Inc.
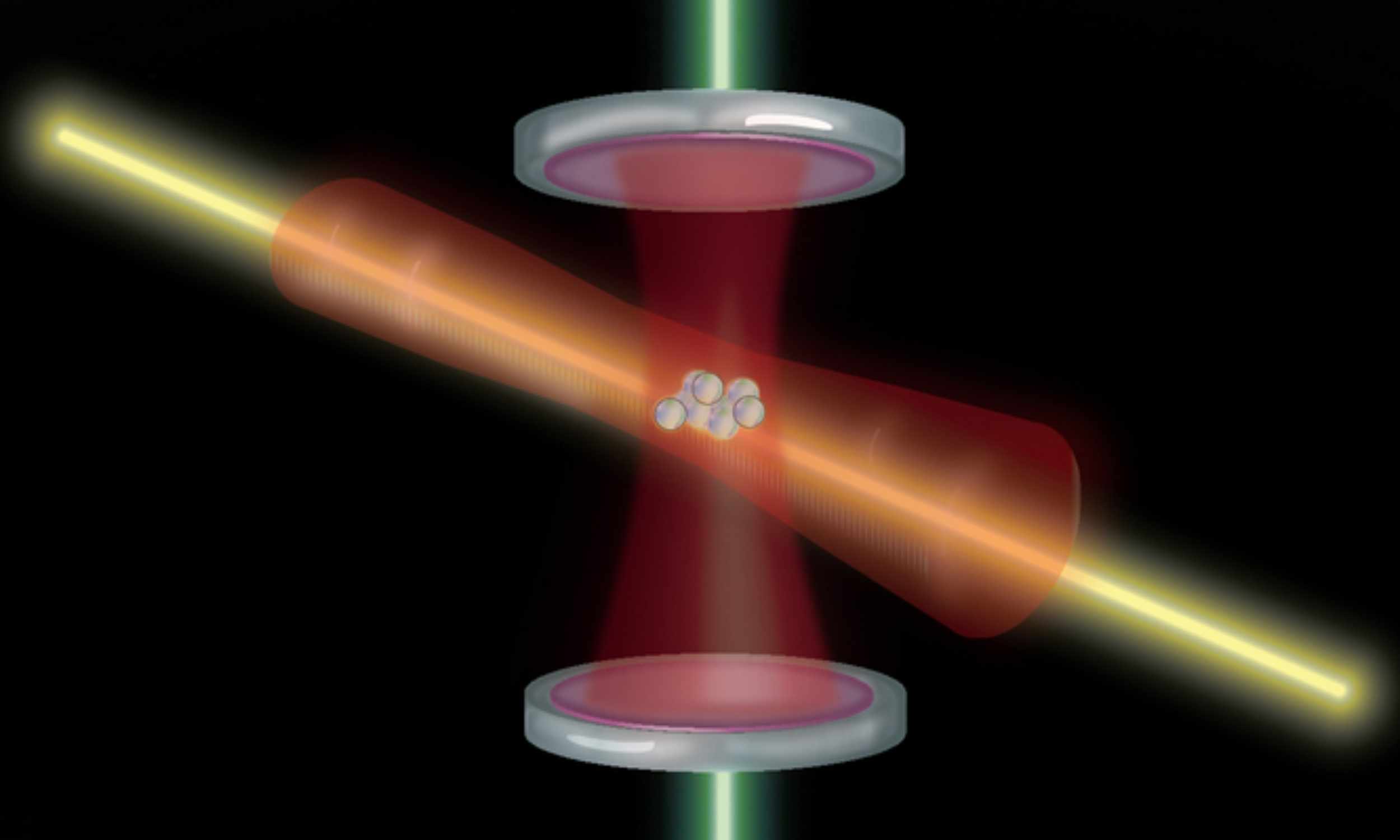
Optical atomic clocks just took a clean step forward. An MIT team reports a method that cuts quantum noise and lets a clock resolve twice as many ticks as before.
The work centers on ytterbium atoms and a new way to steady a laser, and careful…
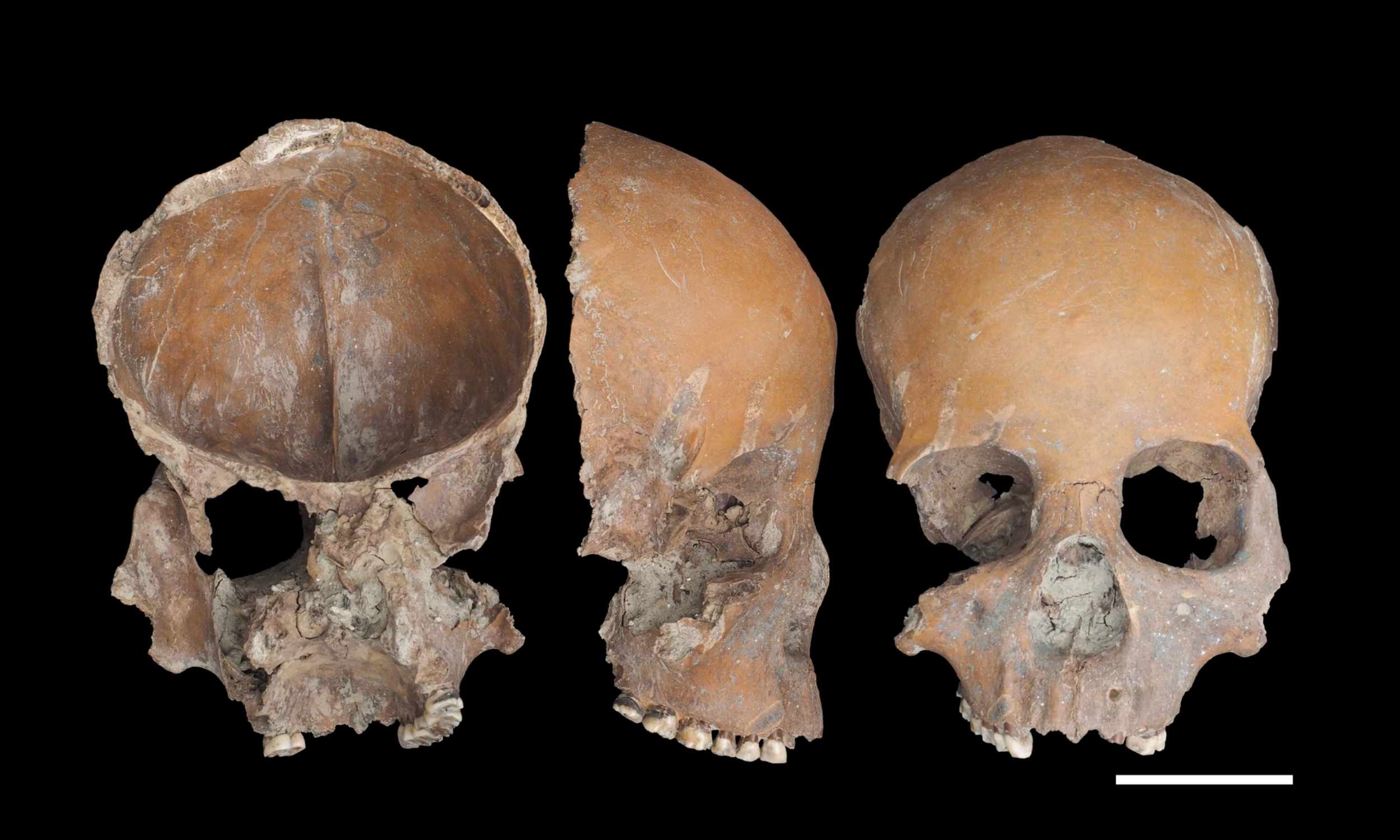
Archaeologists say Neolithic Liangzhu residents shaped human skulls and other bones into everyday tools like bowls, cups, masks, and knives. The dig site dates back roughly 5,000 years and documents the first known example of routine human…
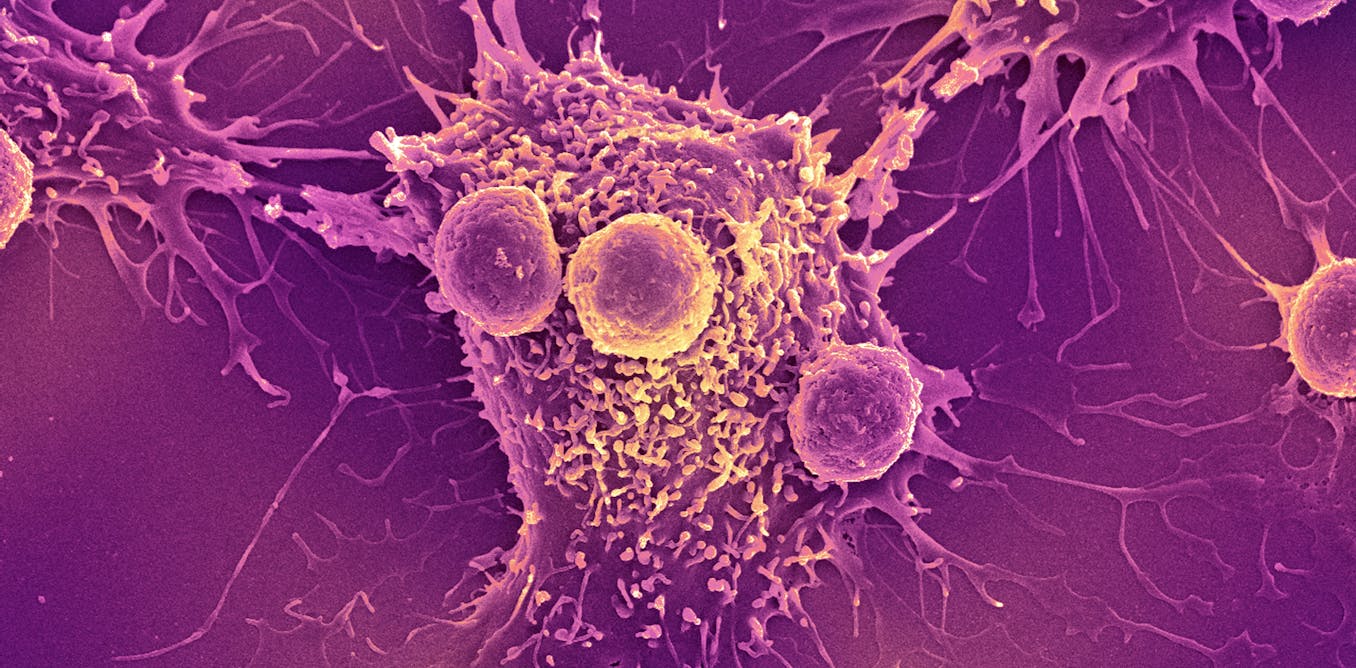
The COVID-19 mRNA-based vaccines that saved 2.5 million lives globally during the pandemic could help spark the immune system to fight cancer. This is the surprising takeaway of a new study that we and our colleagues published in the journal…

Tokyo Olympian Pranati Nayak’s campaign at the World Artistic Gymnastics Championships 2025 in Jakarta came to an abrupt end after she suffered an ankle injury during the qualifying round on Tuesday.
Competing in the women’s vault…

Imagine private, on‑device AI assistants and voice interfaces that run without needing cloud connectivity and respond with minimal latency, chatbots that suggest replies as you type, gaming experiences that adapt in real-time to every player, and smarter always-on, power efficient sensors in wearables and IoT devices that deliver powerful intelligence with low energy use.
These are the kinds of AI experiences that ExecuTorch – Meta’s on-device runtime for PyTorch – and Arm will help developers build, while delivering optimized performance and faster development through a unified PyTorch workflow that runs seamlessly across billions of Arm-based edge devices. The latest milestone for ExecuTorch is today’s General Availability (GA) release, which brings the vision of running AI everywhere into a practical, scalable reality for millions of developers.
The ExecuTorch 1.0 GA release transforms how developers bring their PyTorch models to life at scale. Instead of having model versions, pipelines, or frameworks tuned separately for different device types, developers can author, export, optimize, quantize and deploy the same PyTorch workflow end-to-end across mobile, embedded and edge, minimizing fragmentation and boosting time-to-market.
This gives developers one toolset to seamlessly deploy their apps and workloads, unlocking more advanced, faster AI experiences and features across a broad range of edge devices, from ultra-efficient microcontrollers to flagship smartphones, that run on Arm CPUs, GPUs and Ethos-U NPUs. A recent Meta blog post highlights some examples of on-device AI features powered by ExecuTorch that are already serving billions of people on Facebook, Instagram, Messenger, and WhatsApp, including improved video call quality, music recommendations and creative storytelling.
Together, Arm KleidiAI, CMSIS-NN and the Tensor Operator Set Architecture (TOSA) deliver a unified optimization framework through backend integrations in ExecuTorch, so developers’ apps and workloads targeting Arm-based edge devices automatically benefit from performance and efficiency gains with no need to modify their codes or models.
KleidiAI, which provides Arm kernel integrations to accelerate AI workloads across current and future Arm CPU platforms, is now integrated in multiple frameworks and runtimes, including the XNNPACK Runtime used by ExecuTorch. In parallel, the CMSIS-NN ExecuTorch backend integration serves as the equivalent enabler for Arm Cortex-M-based microcontrollers, providing support for highly efficient, directly integrated inference on constrained edge devices.
The TOSA integration in ExecuTorch provides a unified execution interface for edge AI and machine learning (ML) workloads running on Arm GPUs and Ethos-U NPUs. TOSA converts models into a standardized hardware-agnostic representation, enabling consistent deployment, portability, and verification across these technologies, and reducing engineering effort.
For mobile, the ExecuTorch 1.0 GA release enables developers to deploy more intelligent on-device AI experiences faster and more efficiently across the billions of Arm-based smartphones in use today, as well as next-generation mobile devices.
Key benefits include:
The Arm Ethos-U processor family – which provides best-in-class acceleration across edge AI applications in IoT markets – is a key production backend extensively supported by the ExecuTorch 1.0 GA release.
This delivers:
Moreover, in high performance IoT, the KleidiAI integrations with leading AI frameworks accelerates the performance and efficiency of key models, including Meta Llama 3 and Phi-3, on Arm CPUs.
Learn more about what the ExecuTorch 1.0 GA release means for developers targeting edge AI and high-performance IoT markets in this Arm Community technical blog.
Developers can start benefitting from the ExecuTorch 1.0 GA straight away. Head to developer.arm.com, explore all the learning paths for ExecuTorch, review the relevant documentation and tutorials, and then integrate the workflows into your model export, compilation, and deployment pipelines. Also more details about ExecuTorch can be found on the PyTorch landing page, alongside developer documentation for XNNPACK, Ethos-U, VGF and Vukan devices. Whether building for mobile, PC, wearables or edge sensors, the development path forward is unified and seamless.
The ExecuTorch 1.0 GA release reaffirms Arm’s vision that AI runs consistently and seamlessly across every layer of our hardware ecosystem. Together with the strength of the Arm compute platform and our broad ecosystem, ExecuTorch 1.0 unlocks the scalability, performance, and innovation needed to bring the next generation of edge AI experiences to life everywhere, for everyone.
Visit the Arm talks at the PyTorch conference to learn more about how to deploy AI models and workloads at scale on Arm-based platforms. Visitors can also see ExecuTorch 1.0 in action at the Arm booth and learn more about how to access its full benefits across edge AI applications and workloads.
Any re-use permitted for informational and non-commercial or personal use only.
Melissa Woodbridge
Senior PR Manager
melissa.woodbridge@arm.com
+44 7469 851193

The Magic’s home opener tips off with a matchup against the Heat.
The Orlando Magic play host to the Miami Heat in a Southeast Division showdown to begin each team’s 2025-26 NBA season. From players making debuts for their new squads to…

The Nubia Z80 Ultra is the latest flagship from the brand and it features several improvements over its predecessor, like the latest Snapdragon 8 Elite Gen 5 chipset, a revised camera setup and a larger 7,200mAh Si/C battery with 90W wired…