An emotional Katie Boulter retired from her first-round match at the Hong Kong Open with injury.
The Briton, 29, called for the trainer and was in tears after deciding she could not continue when trailing 20-year-old Filipino Alexandra Eala 6-4…

An emotional Katie Boulter retired from her first-round match at the Hong Kong Open with injury.
The Briton, 29, called for the trainer and was in tears after deciding she could not continue when trailing 20-year-old Filipino Alexandra Eala 6-4…
Updated data from the phase 1/2 OptimUM-01 trial (NCT03947385) shared during the
Investigators reported that the median OS was “notable compared to historical controls,” despite slightly more than one-third (39%) of patients having an ECOG performance status of 1. The median progression-free survival (PFS) with darovasertib plus crizotinib also compared favorably with historical controls. At a median follow-up of 25 months, the median PFS in the respective groups was 7.0 months (95% CI, 3.8-7.7) vs just 2.8 months (95% CI, 2.7-3.4).
The doublet (n = 41) elicited an objective response rate (ORR) of 34.1% (95% CI, 20.1%-50.6%), which was comprised entirely of partial responses; 56.1% of patients achieved stable disease, and 9.8% experienced disease progression. The median duration of response (DOR) was 9.0 months (95% CI, 3.8-12.0). The disease control rate achieved with the combination was 90.2% (95% CI, 76.9%-97.3%), with 85% of patients experiencing any reduction in target lesions.1,2
“These findings suggest that darovasertib plus crizotinib may represent a novel first-line treatment option and support the ongoing registrational phase 2/3 [OptimUM-02 trial (NCT05987332)] in the first-line setting,” Meredith McKean, MD, of Sarah Cannon Research Institute, in Nashville, Tennessee, and colleagues wrote in the presentation.1
It is known that those with metastatic uveal melanoma have a poor prognosis, which median PFS under 3 months and median OS ranging under 1 year. Moreover, the majority of these tumors are known to have PKC-activating mutations in GNAQ/11. Darovasertib targets PKC and has been shown to have activity in this disease. Preclinical evidence supports that crizotinib has complementary activity to darovasertib; as such, investigators sought to evaluate the combination in this population as part of the OptimUM-01 study.
The phase 1/2, multicenter, open-label trial enrolled patients with metastatic uveal melanoma with GNAQ/GNA11 mutations or PRKC fusions who were at least 18 years of age and had an ECOG performance status no higher than 1, measurable disease by RECIST 1.1 criteria, and acceptable organ function. Patients could not have had prior exposure to PKC/MET/GNAQ11 inhibitors in the metastatic setting, nor could they have symptomatic or untreated central nervous system metastases. They were allowed to have previously received ablations, had oligometastatic disease surgically resected, or received neoadjuvant or adjuvant therapy.
In the dose-expansion portion of the research, patients were administered darovasertib at a twice-daily dose of 300 mg and crizotinib at a twice-daily dose of 200 mg. The primary end points for the phase 1 portion were safety, tolerability, and ORR by RECIST 1.1 criteria for the phase 2 portion. Secondary end points included PFS, ORR, and DOR by RECIST 1.1 criteria, quality-of-life measures, and safety.
What has previously been reported?
In April 2023,
When the regimen was given in any line (n = 63), the ORR was 30%; 19 patients experienced a PR. Here, the DCR was 87% and the median PFS was about 7 months. In the group of patients who received the regimen in the first- and any-line but had hepatic-only disease (n = 20), the confirmed ORR was 35%. The DCR was 100% and the median PFS was approximately 11 months.
The median patient age was 64.5 years, with half of patients younger than 65 years and the other half 65 years and older. Most patients were White (93.2%), 52.3% were male, and more than half (61.4%) had an ECOG performance status of 0. Baseline lactate dehydrogenase level was normal for 65.9% of patients. The largest metastatic lesion was no larger than 3.0 cm for 54.5% of patients, 3.1 cm to 8.0 cm for 34.1% of patients, and 8.1 cm or larger for 9.1% of patients. Location of metastases were hepatic only for 52.3% of patients, extrahepatic only for 4.5% of patients, and both for 40.9% of patients. In terms of HLA-A2*02:01 status, 68.2% of patients were negative and 27.3% were positive; this was unknown for 4.5% of patients.
The median dose intensity for darovasertib was 92.6% and 88.0% for crizotinib. Moreover, the mean duration of exposure to darovasertib was 10.0 months. Treatment-related adverse effects (TRAEs) of any grade were experienced by 97.7% of patients who received the doublet; they were grade 3 or higher for 27.3% of patients. Treatment-related serious adverse effects were experienced by 9.1% of patients and were grade 3 or higher for 6.8% of cases. TRAEs resulted in discontinuation for 4.5% of patients.
The most common TRAEs experienced by more than 30% of patients who received the combination were diarrhea (all grade, 90.9%; grade ≥3, 2.3%), nausea (79.5%; 0%), peripheral edema (61.4%; 0%), vomiting (47.7%; 0%), dermatitis acneiform (43.2%; 0%), hypoalbuminemia (43.2%; 2.3%), and fatigue (38.6%; 0%).
“These first reported OS data and broader clinical efficacy observed with a manageable safety profile underscores the potential of the darovasertib and crizotinib combination in the first-line treatment landscape for patients with metastatic uveal melanoma,” Darrin Beaupre, MD, PhD, chief medical officer of IDEAYA Biosciences, stated in a news release.2
In April 2025, the
The phase 2/3 OptimUM-02 trial will evaluate the safety, tolerability, pharmacokinetics, and antitumor activity of darovasertib plus crizotinib in patients with HLA-A*02:01–negative metastatic uveal melanoma.6 The regimen will be compared with investigator’s choice of treatment, which could include pembrolizumab (Keytruda), ipilimumab (Yervoy) plus nivolumab (Opdivo), or dacarbazine.

Kaouther Ben Hania’s Gaza Drama, The Voice of Hind Rajab, has landed a Dec. 17 theatrical release in New York (Film Forum) and Los Angeles (Laemmle Theatres) with a national roll-out to follow via the distribution arm of the pic’s…
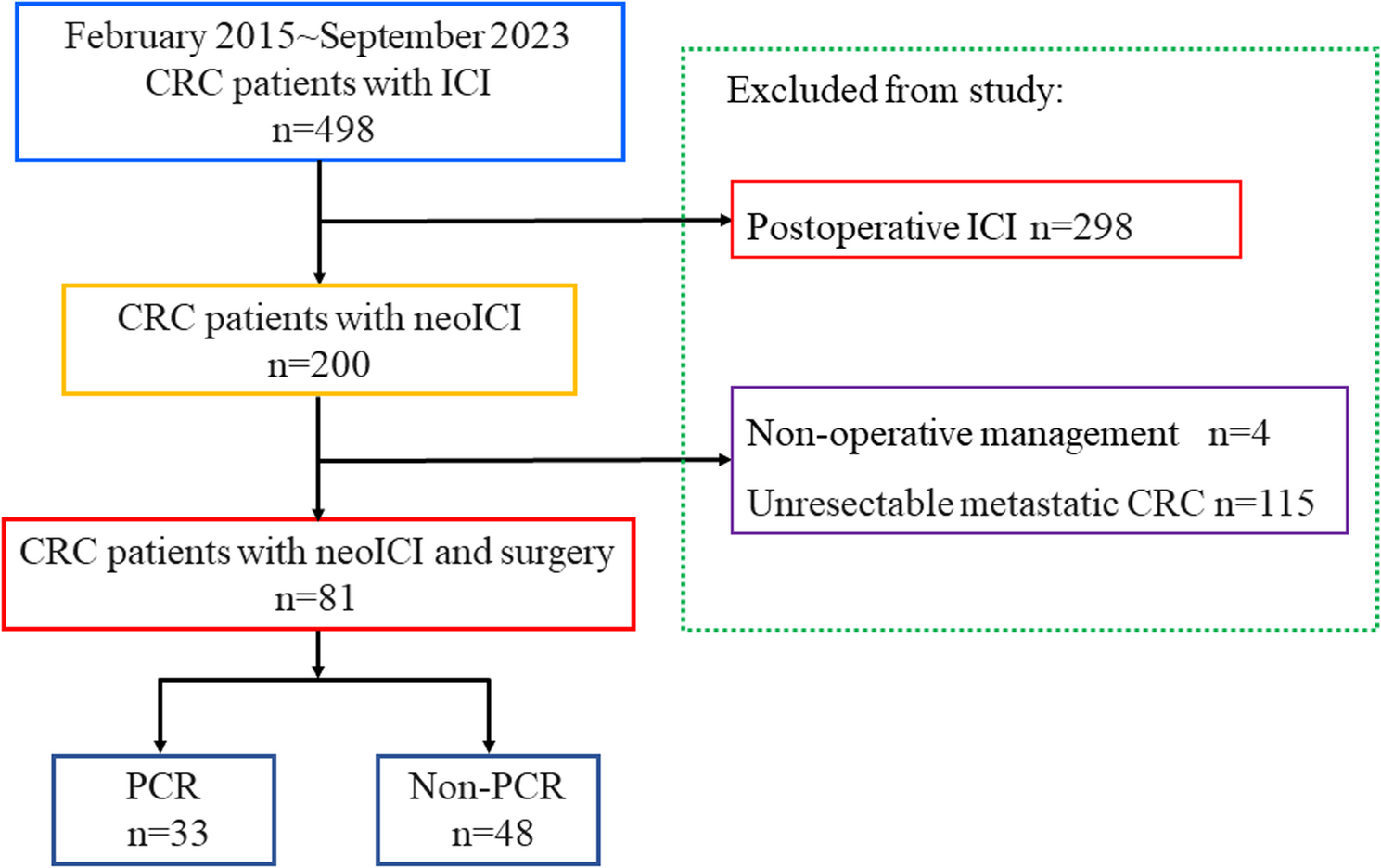
In this single-center retrospective study, all patients received immunotherapy before surgery. After surgery, more than 40% of the patients achieved pCR, with 66.7% and 29.8% of patients with dMMR and pMMR colorectal cancer achieving pCR,…

The Arctic Ocean is losing sea ice, and that is bad news for the climate. Yet a new paper reports a twist that adds nitrogen to these waters, which can feed algae that start the marine food chain.
Researchers measured a steady trickle of nitrogen…

Ibiza’s jewel of the north, Akasha, celebrates its fourth anniversary this weekend.
The weekend kicks off with the huge Namaste Halloween on Friday, 31 October. The line-up is now confirmed, with The Organism, Neliah Kandisha, Maricha and Gaya…
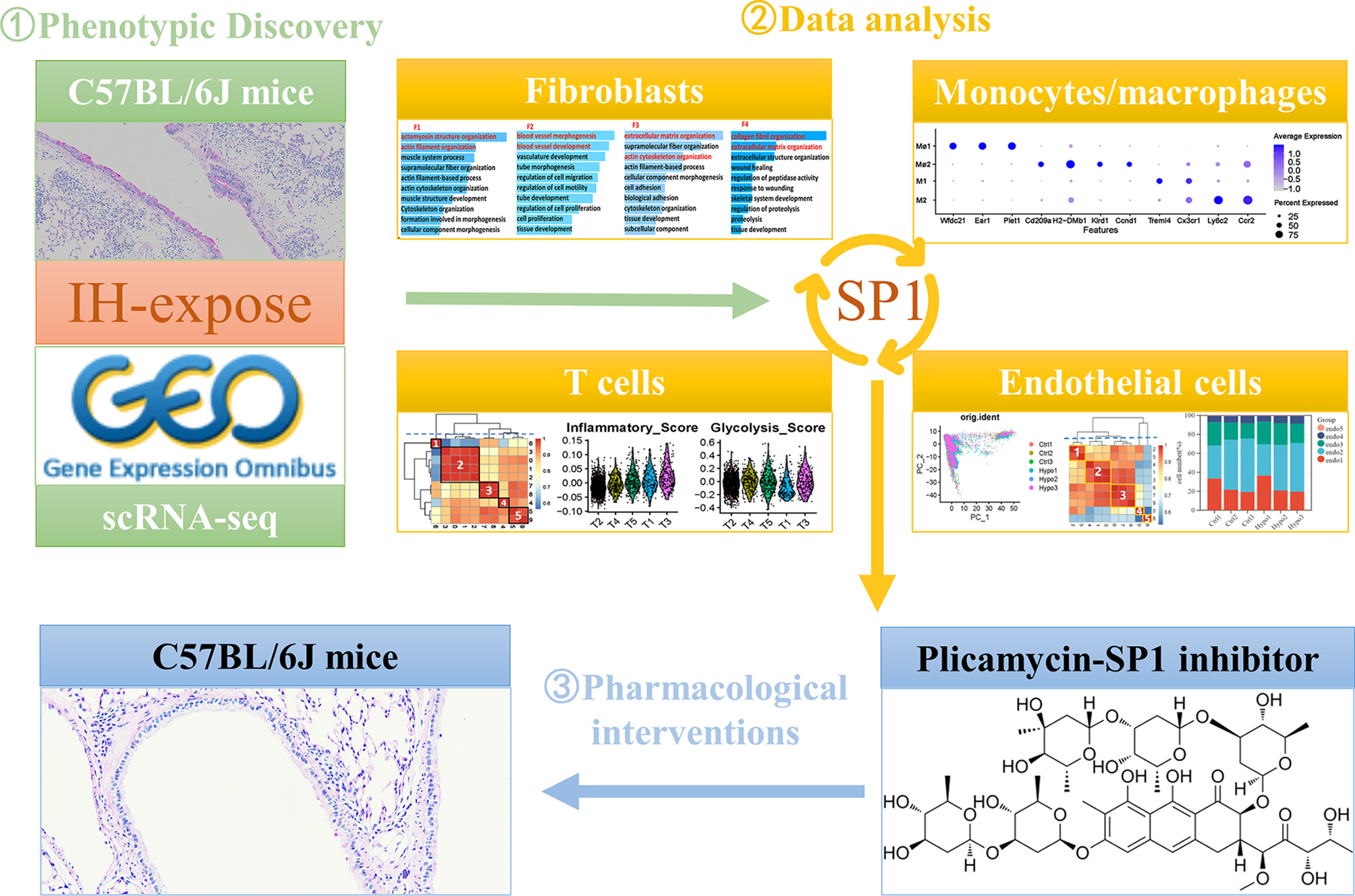
We exposed C57BL/6J mice to either IH or normoxia for 6 weeks to investigate the effects of IH on lung tissue. After the exposure period, lung tissues were collected for histopathological staining and analysis. Compared with control mice, those exposed to IH exhibited significant increases in pulmonary inflammation, mucus staining, and collagen deposition (Fig. 1A-D). Phalloidin staining revealed enhanced cytoskeletal organization and increased stress fiber formation, which may reflect actin remodeling associated with both structural cells and infiltrating inflammatory cells in the lungs of IH-exposed mice (Fig. 1A, E). Additionally, histochemical staining revealed increased expression of Cd11b, Mpo, and Cd68 in the lungs of IH-exposed mice, indicating that IH exposure promoted infiltration of immune cells. The upregulation of Cd31 expression in lung tissue further confirmed that IH exposure stimulated remodeling of pulmonary blood vessels (Fig. 1F-G). In summary, IH exposure induced pronounced inflammatory infiltration and structural alterations, contributing to extensive pulmonary tissue and vascular remodeling in mice.
Pathology of IH-exposed mouse lung tissue (n = 6). (A) Representative images of H&E and PAS staining, and Masson’s trichrome (10× objective) and phalloidin staining (20× objective) in mouse lung tissue. Evaluation of (B) the pulmonary airway inflammation score, (C) collagen deposition, (D) number of PAS+ cells in the airways, (E) remodelling of F-actin in lung tissue in mouse lung tissues.(F) Assessment of the level of pulmonary microvascular remodelling. (G) Immunohistochemical staining for Cd11b, Mpo, Cd68 and Cd31 in the mouse lungs (20× objective). IH, intermittent hypoxia; PAS, periodic acid–Schiff; Cd11b, cluster of differentiation 11b; MPO, myeloperoxidase; Cd68, cluster of differentiation 68; Cd31, cluster of differentiation 31. The data are presented as the mean ± SD. Statistical significance was assessed using Student’s t tests. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Next, we retrieved scRNA-seq data from the GEO database, including lung tissue samples from both IH model mice (n = 3) and healthy control mice (n = 3), to gain a comprehensive understanding of the cellular landscape in the lung tissue of IH model mice (Supplementary Table 1). After rigorous quality control and filtering, a total of 48,272 cells were retained from the scRNA-seq data for downstream analysis (Supplementary Fig. 1 and Supplementary Table 1). In the scRNA-seq dataset, the median number of unique molecular identifiers (UMIs) per cell was 1978, and the median number of genes detected per cell was 996. After PCA-mediated reduction of these high-quality data, the first 15 PCs were included in the follow-up analysis (Supplementary Fig. 1). We applied the Harmony algorithm to each dataset again to minimize batch effects between different scRNA-seq datasets. Subsequently, we adopted a clustering method based on t-SNE to initially identify 12 clusters according to typical markers of different cell types. These cell types include type 1 alveolar epithelial cells (AT1), type 2 alveolar epithelial cells (AT2), macrophages/monocytes, megakaryocytes, B cells, T cells, fibroblasts, endothelial cells, eosinophils, natural killer (NK) cells, neutrophils, and erythroid cells (Fig. 2A-B, Supplementary Table 2). These cells were present in each sample (Fig. 2E and Supplementary Fig. 1). The specific number of cells identified in each sample is provided in Supplementary Table 2. A total of 195 cells in Clusters 19 and 21 carried markers of two cell types whose expression differed significantly at the same time. We conducted a secondary analysis and divided these 195 cells into three clusters to exclude the possibility of insufficient dimensionality reduction (Fig. 2C). A total of 154 cells in Clusters 0 and 2 expressed endothelial cell and B-cell markers simultaneously. Forty-one cells in Cluster 1 expressed both B-cell and T-cell markers (Fig. 2D-F). We decided not to include these double-positive cells in subsequent analyses to ensure the accuracy and reliability of our results. The calculation of the cell proportion for each cell type indicated significant changes (Fig. 2G). Among all the cells collected, endothelial cells accounted for the largest proportion and exhibited the most obvious increase, and macrophages presented the greatest change among immune cells. Consistent with previous reports, IH exposure increased the number of type 1 alveolar epithelial cells [21]. Notably, IH also promoted the aggregation of fibroblasts in the lung (Fig. 2H) [22, 23].
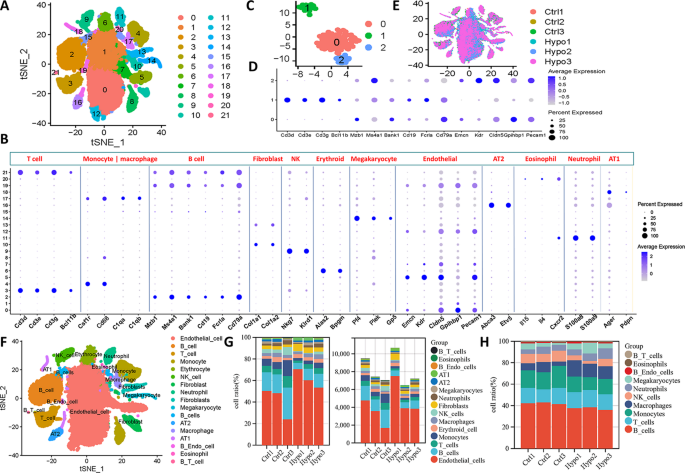
A single-cell landscape of IH-exposed mouse lungs. (A) t-SNE dimensionality reduction analysis of all lung parenchymal cells. (B) Bubble maps showing marker genes of different cell clusters. The colour depth of the dots indicates the level of gene expression, and the size of the dots indicates the percentage of cells expressing the corresponding gene. (C) Redimensionality reduction and (D) cluster marker genes for the 19 and 21 cell clusters. (E) Distribution of different cell clusters in different samples. (F) Manual annotation of the cell types to which different cell clusters belong. Different colours indicate different cell types. (G) Proportions and specific numbers of different types of cells in different samples. (H) Proportions of different types of immune cells in different samples. t-SNE, t-distributed stochastic neighbour embedding; AT1, alveolar type 1 cell
Fibroblast heterogeneity was analyzed in scRNA-seq data from six samples (Fig. 3A). Reclustering revealed four fibroblast subtypes (F1-F4) with distinct gene expression profiles (Fig. 3B-C). Acta2+ fibroblasts (F1) expressed high levels of smooth muscle contraction genes such as Actc1 and Myh11 (Fig. 3E), consistent with myoid fibroblasts described previously [24]. GO analysis showed enrichment in actomyosin and muscle system processes for F1 (Fig. 3D). Tcf21+ fibroblasts (F2) highly expressed vascular development genes (Fig. 3E), with GO terms related to blood vessel morphogenesis (Fig. 3D). Dcn+ fibroblasts (F4) expressed extracellular matrix (ECM) genes (Col1a1, Col3a1) and were enriched in ECM organization pathways (Fig. 3D-E). A proliferative fibroblast cluster (F3) was identified by high ribosomal gene expression and low apoptosis gene expression (Fig. 3E).
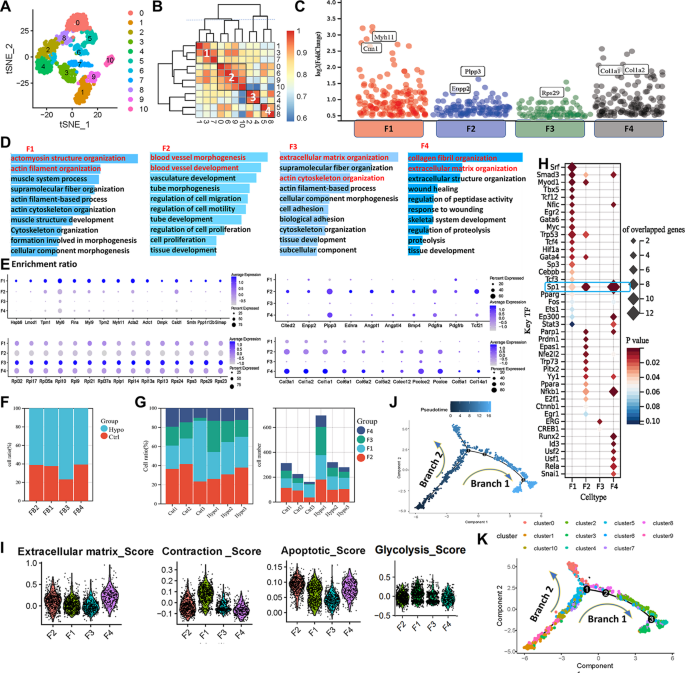
Characterization of fibroblasts in the lungs of IH-exposed mice. (A) Redimensionality analysis of fibroblasts. (B) Correlation analysis of different cell clusters. F1 = Cluster 1 + Cluster 3 + Cluster 7, F2 = Cluster 0 + Cluster 6 + Cluster 9 + Cluster 10, F3 = Cluster 2 + Cluster 4, F4 = Cluster 5 + Cluster 8. (C) The differential expression analysis indicated the significantly upregulated genes for each fibroblast subtype (adjusted p value < 0.05 & Log FC > 0.5). Cell marker (E) and functional enrichment (D) analyses of different fibroblast subtypes. The colour depth of the dots indicates the level of gene expression, and the size of the dots indicates the percentage of cells expressing the corresponding gene. (F) Proportions of different fibroblast subtypes in different experimental groups. (G) Proportions and specific numbers of different types of cells in different samples. (H)Bubble map showing the number of predicted transcription factors regulating signature target genes in fibroblast subtypes. (I) Violin plot of characteristic scores among different fibroblast subtypes. Pseudotime distribution (J) and analysis of the differentiation trajectory (K) of different fibroblast clusters. t-SNE, t-distributed stochastic neighbour embedding; F1, myoid fibroblasts; F2, vascular fibroblasts; F3, persistent fibroblasts; F4, matrix fibroblasts
Compared to controls, all four fibroblast subtypes expanded after IH exposure, with the most significant increases seen in F1 and F2 cells (Fig. 3F-G). Through transcription factor target analysis, we characterized the predominant regulatory networks in each fibroblast subtype. In F2 and F4 cells, Sp1—a transcription factor involved in transcriptional activation—was predicted to regulate numerous target genes. F1 cells were predominantly regulated by serum response factor (Srf), a well-known transcription factor implicated in the contractile phenotype of vascular smooth muscle cells (Fig. 3H) [25]. In F3 cells, Erg was identified as a key regulator, consistent with its known role in fibroblast activation [26]. Gene set scoring revealed F1 fibroblasts had the highest contractility score, F4 fibroblasts the highest ECM score, and F3 the lowest apoptosis score (Fig. 3I). Glycolytic activity scores did not differ significantly among the subtypes. During disease progression, significant proportional changes in fibroblast subtypes were observed. Trajectory analysis showed that F1 and F2 cells were evenly distributed along the developmental timeline, while F4 cells were mainly present during the intermediate stages, and F3 cells were predominantly enriched at the late stages. These trends may explain the greater incidence of hypertension and pulmonary fibrosis in OSAHS patients than in the general population(Fig. 3J-K, Supplementary Fig. 2) [27, 28].
The t-SNE analysis grouped monocytes and macrophages into 14 clusters based on 2,000 highly variable genes (Fig. 4A, Supplementary Fig. 3). Clusters 0, 5, and 8 were annotated as macrophage subtype 1 (Mφ1), marked by Wfdc21, Ear1, and Plet1. Clusters 2, 10, 11, and 12 formed monocyte subtype 1 (M1), expressing Treml4 and Cx3cr1, corresponding to nonclassical monocytes. Clusters 3, 4, 6, 7, and 13 were macrophage subtype 2 (Mφ2), marked by Cd209a, H2-Dmb1, Klrd1, and Ccnd1. Clusters 1 and 9 were monocyte subtype 2 (M2), expressing Ly6c2 and Ccr2, corresponding to classical monocytes (Fig. 4B-C).
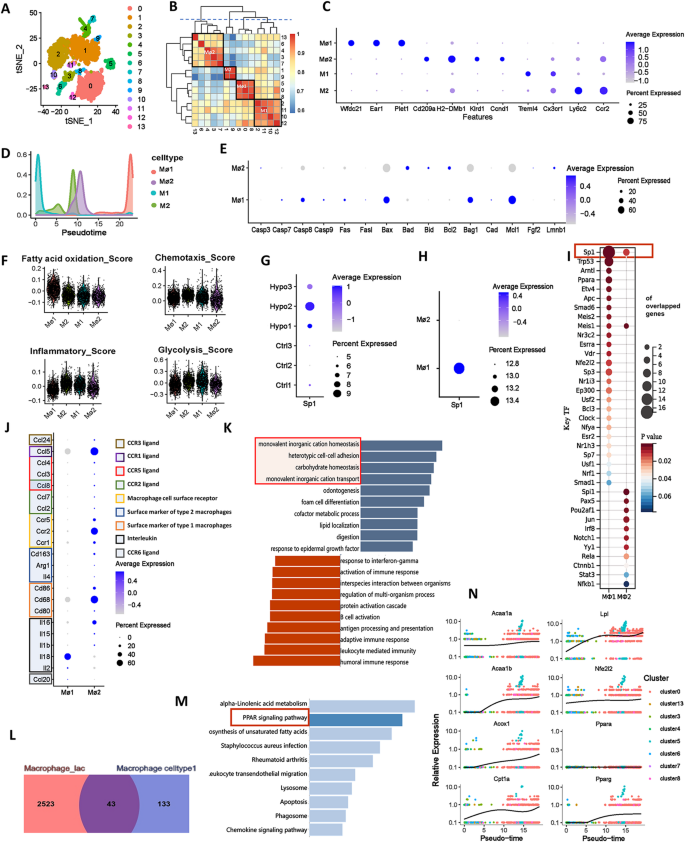
Characterization of different subtypes of monocytes and macrophages in IH-exposed mouse lungs. (A) t-SNE diagram of monocyte and macrophage redimensionality reduction. (B) Correlation analysis of different cell clusters. (C) Bubble map showing the marker genes of different cell subtypes. The colour depth of the dots indicates the level of gene expression, and the size of the dots indicates the percentage of cells expressing the corresponding gene. (D) Curves showing changes in the distributions of different subtypes of monocytes and macrophages over time. (E) Bubble maps of the expression levels of apoptosis-related genes in different subtypes of monocytes and macrophages. (F) Violin plot of characteristic scores among different monocyte and macrophage subtypes. (G) Bubble map of the amount of Sp1 expressed in different samples. (H) Bubble map of Sp1 expression in monocytes and macrophages. The colour depth of the dots indicates the level of gene expression, and the size of the dots indicates the percentage of cells expressing the corresponding gene. (I) Bubble map showing the number of predicted transcription factors regulating signature target genes of monocytes and macrophages. (J) Cytokine expression levels in different subtypes of macrophages. GSEA (K) of Mφ1 characteristic genes. Dark blue represents an FDR < 0.05, and light blue represents an FDR > 0.05 but p < 0.05. Venn diagram (L) and KEGG enrichment analysis (M) of the intersections between Mφ1 signature genes and significantly upregulated genes in lactate-stimulated macrophages. (N) Analysis of the developmental trajectory of genes related to the Ppar signalling pathway. t-SNE, t-distributed stochastic neighbour embedding; M1, monocyte subtype 1; Mφ1, macrophage subtype 1; M2, monocyte subtype 2; Mφ2, macrophage subtype 2
Compared to controls, the proportion of M1 cells decreased while Mφ1 cells increased significantly. Pseudotime analysis indicated that monocytes progressed from M1 to M2, then to Mφ2 and ultimately Mφ1, supporting enhanced monocyte-to-macrophage transition in the lungs under IH exposure (Fig. 4F, Supplementary Fig. 4). Functional scoring revealed that Mφ1 exhibited the highest fatty acid oxidation and lowest inflammation levels among the four subtypes, while M2 showed the strongest chemotaxis, likely due to recruitment by inflammatory signals. Both macrophage subtypes had lower glycolysis levels than monocytes, and Mφ1 displayed disordered apoptosis-related gene expression, suggesting terminal differentiation (Fig. 4E-F). Transcription factor analysis indicated that Sp1 predominantly regulated Mφ1-related genes, with higher expression in the IH group (Fig. 4G-I). GO enrichment showed that M1 was associated with innate immune responses, M2 with immune regulation, Mφ2 with lymphocyte activation, and Mφ1 with lipid metabolism and endocytosis (Supplementary Fig. 4H-K).
Additionally, we analyzed cytokine expression in Mφ1 and Mφ2 from the lungs of IH-exposed mice. The results showed that Mφ1 is characterized by a high expression level of Il18, whereas Mφ2 displayed higher levels of Ccl5, Ccr2, and Cd68, indicative of a classical pro-inflammatory phenotype (Fig. 4J). GSEA of Mφ1 signature genes linked them to processes such as monovalent inorganic cation homeostasis, cell adhesion, and carbohydrate metabolism (Fig. 4K), indicating roles in ion balance and metabolic regulation. KEGG analysis showed significant enrichment of PPAR-related pathways in Mφ1 (Supplementary Fig. 4L), highlighting PPAR’s role in metabolism and immune function. Using lactate-treated macrophage data (GSE115354), we found 43 overlapping upregulated genes enriched in PPAR pathways (Fig. 4L-M), confirming PPAR’s involvement in IH-induced Mφ1 changes. Expression of key PPAR pathway genes (Acaa1a, Acaa1b, Acox1, Cpt1a, Lpl, Nfe2l2, Pparg) increased over pseudotime (Fig. 4N), indicating enhanced fatty acid oxidation and lipid metabolism in macrophages after IH exposure.
The t-SNE analysis of scRNA-seq data from six samples revealed the heterogeneity of T cells and identified nine distinct subtypes through clustering (Fig. 5A and B). By calculating the similarity between cell clusters (Fig. 5C), we further classified these clusters into five major subtypes (Fig. 5D). Subtype T1 (Cluster 6) corresponds to exhausted CD8+ T cells, characterized by markers of chronic antigen stimulation and functional exhaustion. Subtype T2 (Clusters 0, 1, 2, 3) represents memory CD8+ T cells with high Cd8a and Ccr7 expression, involved in antigen recognition and immune surveillance. Subtype T3 (Clusters 7, 8) is marked by elevated Gata3, Ccr2, and Cxcr6, consistent with CD4+ Th2 cells that regulate allergic inflammation. Subtype T4 (Cluster 4) shows effector memory features, with Ccl5, Cd8a, and Cxcr3 expression indicating strong migratory and antiviral capacity. Subtype T5 (Clusters 5, 9) comprises regulatory T cells (Tregs), characterized by Foxp3, Ctla4, and Lcos expression, crucial for immune tolerance and suppressing excessive immune responses.
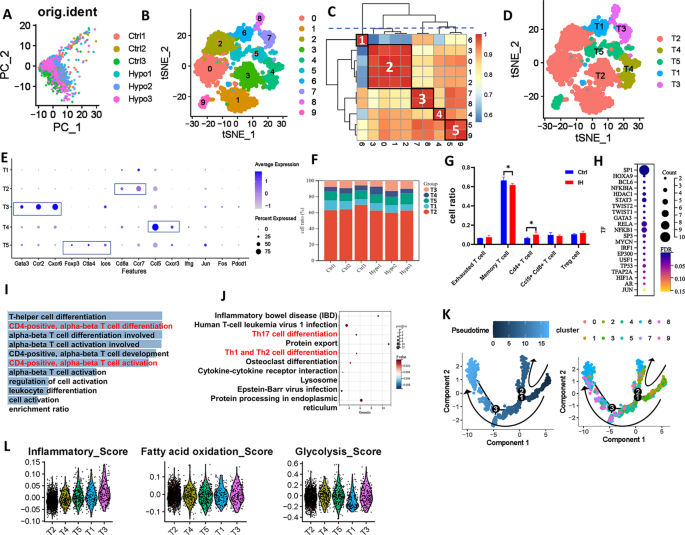
Characterization of different subtypes of T cells in IH-exposed mouse lungs. (A) PCA diagram of T-cell redimensionality reduction. (B) t-SNE diagram of T-cell redimensionality reduction. (C) Correlation analysis of different cell clusters. (D) t-SNE distribution of different subtypes of T cells. (E) Bubble map showing the marker genes of different T-cell subtypes. (F) and (G) Distribution of different T-cell subtypes in different samples. (H) Bubble map showing the number of predicted transcription factors regulating signature target genes of T3 cell. KEGG (I) and GO enrichment analyses (J) of highly expressed genes in T3 cells. (K) Analyses of the differentiation trajectory and pseudotime distribution of different clusters of T cells. (L) Violin plot of the characteristic scores among different T-cell subtypes. PCA, principal component analysis; t-SNE, t-distributed stochastic neighbour embedding
After IH exposure, the proportion of memory T cells (T2) decreased significantly, while CD4+ Th2 cells (T3) increased (Fig. 5F-G). Notably, SP1, a transcriptional regulator linked to T3 signature genes, was significantly upregulated in the IH group, suggesting its role in T3 cell regulation (Fig. 5H). KEGG and GO analyses of T3 highly expressed genes showed enrichment in pathways related to CD4-positive, alpha-beta T-cell differentiation and activation, including Th17 and Th1/Th2 differentiation (Fig. 5I-J). Trajectory analysis showed that T1 and T2 cells were evenly distributed along the developmental timeline, while T4 cells were mainly enriched during the intermediate-to-late stages, and T3 cells were predominantly present at the late stage (Fig. 5L). T3 cells exhibited the highest inflammation score, indicating that their activation and cytokine secretion may exacerbate pulmonary inflammation in late-stage IH (Fig. 5K). Fatty acid oxidation was similar across subtypes, while T1 cells displayed markedly lower glycolysis, consistent with an exhausted phenotype. These findings demonstrate that IH reshapes T-cell composition and function, promoting Th2-skewed inflammation and T-cell exhaustion.
We analyzed endothelial cells from six samples using scRNA-seq data. t-SNE analysis identified nine clusters (Fig. 6A), which were grouped into five subtypes based on cluster similarity (Fig. 6B): endo1 (Clusters 1 and 5), endo2 (Clusters 1, 3, and 4), endo3 (Clusters 6, 7, and 9), endo4 (Cluster 0), and endo5 (Cluster 8). The distribution of these subtypes across samples is shown in Fig. 6C and D. Marker gene analysis revealed distinct profiles: Cyb5r3 and Rps29 for endo1; Cldn5 and Foxf1 for endo2; Emp2 and Cdh5 for endo3; Vwf and Cytl1 for endo4, consistent with venous endothelial cells [29]; and Ccl21a and Mmrn1 for endo5, matching lymphatic endothelial cell signatures (Fig. 6E) [29]. Among these, endo2 was the most abundant, while endo5 was the least prevalent. Notably, IH exposure increased the proportions of endo1 to endo3 cells (Fig. 6F). Pseudotime trajectory analysis revealed a dynamic transition of endothelial subtypes over time: endo2 and endo5 predominated in the early stages, endo3 was evenly distributed throughout the trajectory, and endo1 and endo4 became dominant at the terminal stage (Fig. 6G, Supplementary Fig. 5). KEGG enrichment analysis demonstrated subtype-specific functional programs: endo1 was enriched in “ribosome” and “oxidative phosphorylation”; endo2 in “antigen processing and presentation” and “fluid shear stress and atherosclerosis”; endo3 in “IL-4/IL-13 signaling” and “apoptosis”; endo4 in “TGF-beta signaling” and “stem cell pluripotency”; and endo5 in “ferroptosis” and “autophagy” (Fig. 6H). These findings suggest that during IH exposure, endothelial cells undergo functional reprogramming from immune and stress responses toward a more reparative and metabolically active phenotype.
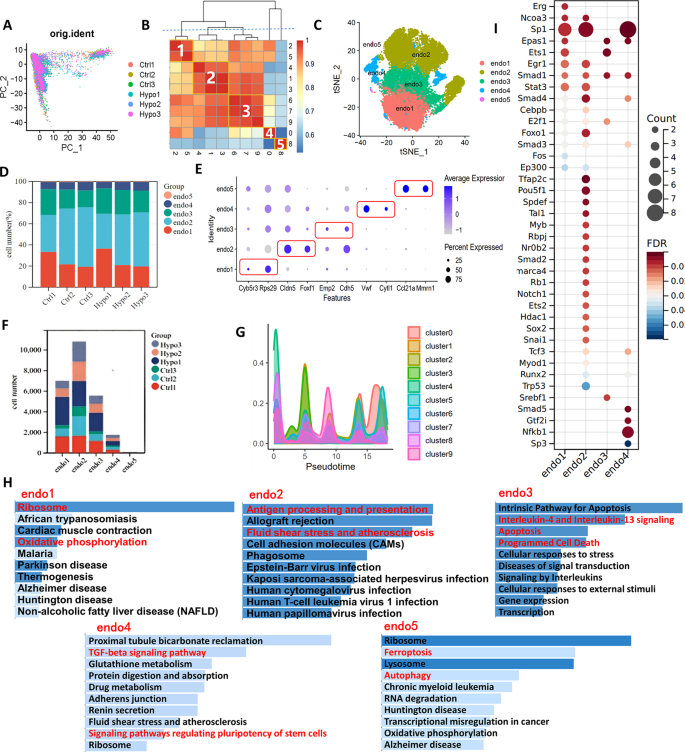
Characterization of different subtypes of endothelial cells in IH-exposed mouse lungs. (A) t-SNE diagram of endothelial cell redimensionality reduction. (B) Correlation analysis of different endothelial cell clusters. Endo1 = Cluster 2 + Cluster 5, endo2 = Cluster 1 + Cluster 3 + Cluster 4, endo3 = Cluster 6 + Cluster 7 + Cluster 9, endo4 = Cluster 0, endo5 = Cluster 8. (C) t-SNE distribution of different subtypes of endothelial cells. (D) The distribution of different endothelial cell subtypes in different samples. (E) Bubble map showing the marker genes of different endothelial cell subtypes. (F) The specific numbers of different endothelial cell subtypes in different samples. (G) Curves showing the distributions of different subtypes of endothelial cells over time. (H) KEGG enrichment analysis of characteristic genes of different endothelial cell subtypes. (I) Bubble map showing the number of predicted transcription factors regulating signature target genes of different endothelial cell subtypes. PCA, principal component analysis
We analyzed receptor‒ligand interactions between monocytes/macrophages and other altered cell types to further elucidate the cellular interactions within lung tissue exposed to IH. The results revealed that the interactions between the endo2 and other cell subtypes were particularly strong. Among these interactions, endo2 cells exhibited similarly strong interactions with both Mφ1 and M2 , and these interactions were markedly stronger than those observed with other cell types (Fig. 7A and B). Additionally, interactions between Tgfb1–(Tgfbr1 + Tgfbr2), Ccl6–Ccr2, Sema3a–(Nrp1 + Plxna2), and Sema3c–(Nrp1 + Plxna2) are highly probable within the network of monocytes/macrophages and other altered cell types, with Ccl6–Ccr2 showing the strongest potential for interaction. Notably, the receptor for Ccl6, Ccr2, was expressed in multiple cell subtypes, with prominent expression in endothelial cells and macrophage/monocyte-related subtypes (Fig. 7E). We performed immunohistochemistry to investigate this phenomenon further, and the results confirmed that Ccr2 expression was indeed upregulated in the lung tissue of the IH-exposed group (Fig. 7F), suggesting that Ccr2 may play a significant role in the pathophysiology of IH.
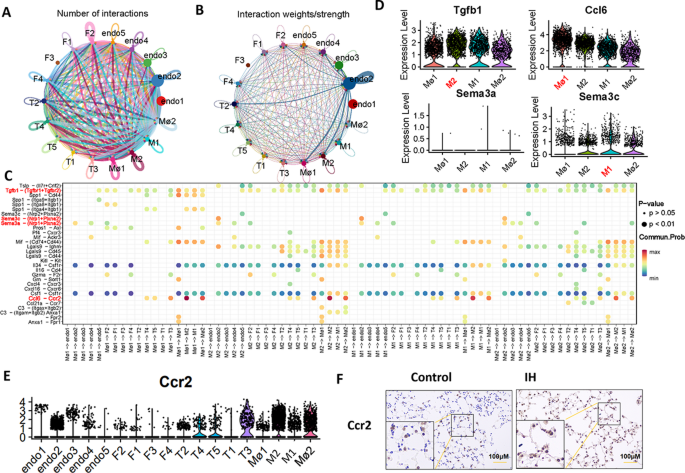
Analysis of interactions between different cell subtypes. (A, B and C) Receptor‒ligand interactions between monocytes, macrophages, endothelial cells, and fibroblast subtypes. (D) Violin plot showing the expression levels of Tgfb1, Ccl6, Sema3a and Sema3c in different monocyte/macrophage subtypes. (E) Violin plot showing the expression levels of Ccr2 in different cell types. (F) Immunohistochemical staining for Ccr2 in the mouse lungs (20× objective). IH, intermittent hypoxia; Ccr2, C-C chemokine receptor type 2
In this study, we first confirmed that SP1 expression was significantly upregulated in the lungs of mice exposed to IH, as evidenced by both immunofluorescence staining and western blot analysis (Fig. 8A-D). Based on this observation, we established an IH mouse model combined with the SP1 inhibitor plicamycin to further investigate its therapeutic potential. Western blot analysis and immunofluorescence analysis (Fig. 8E-F and H-I) showed that plicamycin treatment partially reversed the IH-induced upregulation of SP1 in lung tissue. Similarly, measurement of lung hydroxyproline levels suggested a modest reduction following plicamycin treatment compared with IH alone (Fig. 8G). Subsequently, histopathological evaluation revealed that plicamycin tended to attenuate IH-induced pulmonary inflammation, mucus secretion, and collagen deposition (Fig. 8H and J–L), indicating that SP1 plays a central role in mediating lung injury under IH exposure. Additionally, phalloidin staining revealed increased F-actin remodeling and stress fiber formation in the lungs of IH-exposed mice, which was partially alleviated by plicamycin treatment (Fig. 8H and M). Furthermore, PCR analysis revealed that plicamycin effectively reduced the expression of genes involved in inflammation and fibrosis, including Fizz1, Ym1, Il6, α-Sma, Cd68, Ccl2, Cd80, Ccl7, Col1a1, and iNOS (Supplementary Fig. 6 A), further suggesting that SP1 plays a key role in pulmonary inflammation and fibrosis induced by IH exposure. In conclusion, the SP1 inhibitor plicamycin significantly suppresses the inflammatory response, cytoskeletal remodeling, and fibrosis in the lung tissue of IH-exposed mice.
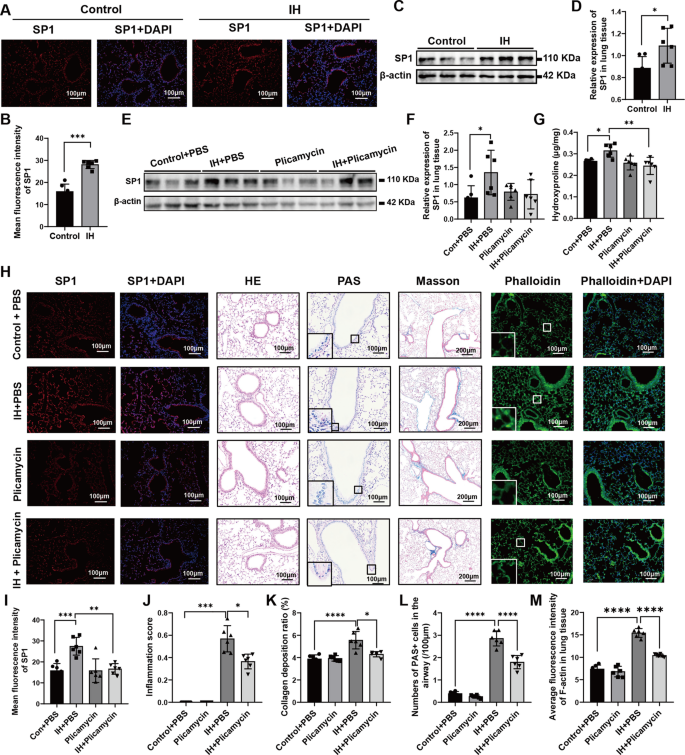
SP1 expression and the effect of plicamycin on lung tissue in mice exposed to intermittent hypoxia (n = 6). (A) Immunofluorescence staining of SP1 (red) and nuclei (blue, DAPI) in lung tissues from Control and IH groups. (B) Quantification of mean fluorescence intensity of SP1 in (A). (C) Western blot analysis of SP1 protein expression in lung tissues from Control and IH groups, with β-actin as a loading control. (D) Quantitative analysis of relative SP1 protein expression in (C). (E) Western blot analysis of SP1 protein expression in lung tissues from Control + PBS, IH + PBS, Plicamycin, and IH + Plicamycin groups, with β-actin as a loading control. (F) Quantitative analysis of relative SP1 protein expression in (E). (G) Measurement of hydroxyproline content in lung tissues from different groups. (H) Representative images showing SP1 expression (immunofluorescence, SP1 in red, DAPI in blue), HE staining, PAS staining, Masson’s trichrome staining (10× objective, others 20× objective), and phalloidin staining (phalloidin in green, DAPI in blue) in lung tissues from four groups (Control + PBS, IH + PBS, Plicamycin, IH + Plicamycin) (objective magnifications: 10× or 20×, as indicated). (I) Quantification of mean fluorescence intensity of SP1 in (H). (J) Airway inflammation score based on HE staining. (K) Collagen deposition ratio assessed by Masson’s trichrome staining. (L) Number of PAS⁺ cells in the airways. (M) Average fluorescence intensity of F-actin (phalloidin staining) in lung tissue. IH, intermittent hypoxia; HE, haematoxylin and eosin; PAS, periodic acid–Schiff; The data are presented as the mean ± SD. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test as post hoc analysis. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
GBD 2017 risk Factor Collaborators. Global, regional, and National comparative risk assessment of 84 behaviournvironmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic…

George Ford has been named at fly-half for England’s opening autumn Test against Australia on Saturday at Allianz Stadium.
Northampton’s Fin Smith drops to the bench and there is no space in the 23-man squad for Harlequins playmaker Marcus Smith.
Continue Reading

ASCAP has revealed its lineup of honorees for the 17th annual Women Behind The Music event, taking place Nov. 12 in Los Angeles.
Kehlani, Kali Uchis and “KPop Demon Hunters’” EJAE will be recognized by the ASCAP Membership team as…