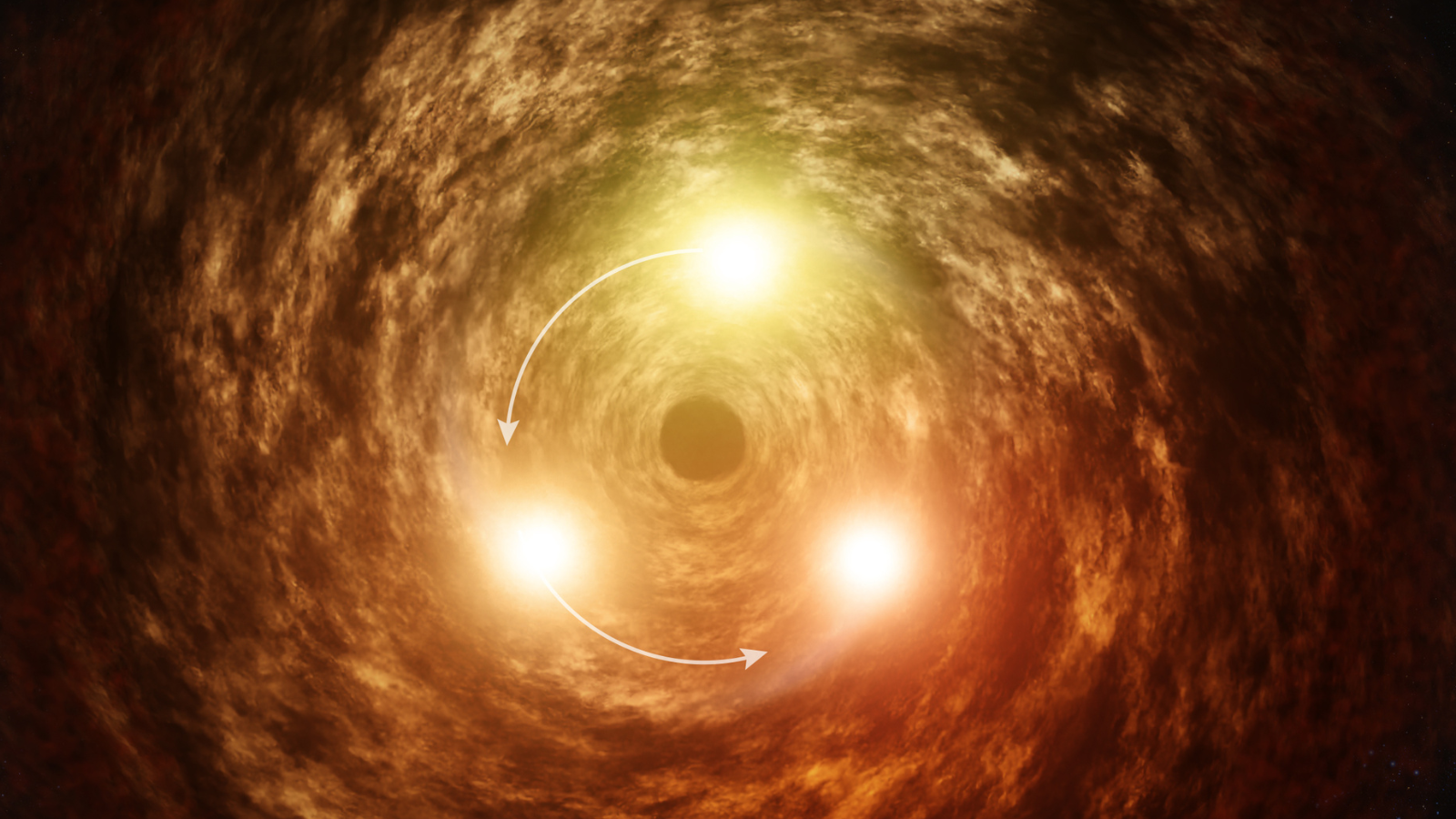
Astronomers have used the James Webb Space Telescope (JWST) to observe flares from Sagittarius A*, the supermassive black hole at the heart of the Milky Way, in a new light. The new modelling of these observations could help scientists get to…
James Webb Space Telescope watches our Milky Way galaxy’s monster black hole fire out a flare