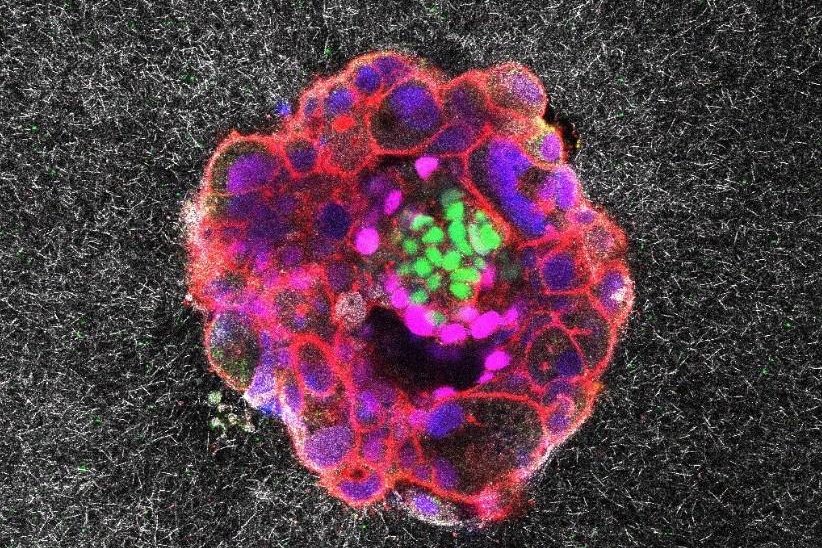
1 of 4 | A human embryo is shown implanting itself inside a simulated uterine wall in an image taken from the first real-time video of the process ever recorded. Spanish researchers say they hope their video will lead to a deeper understanding of infertility. Photo courtesy Institute for Bioengineering of Catalonia
Aug. 11 (UPI) — A team of Spanish researchers announced Friday they have for the first time recorded video of a human embryo implanting itself in a simulated uterine wall, revealing never-before-seen details of how 5-day-old embryos carry out the mysterious process.
Using advanced microscopy techniques allowing the scientists to record the human embryo in full color and 3D, the “astonishing” videos provide the first-ever, real-time glimpse of the implantation process and have provided key insights into how it actually works, they said.
Researchers from the Institute for Bioengineering of Catalonia and Dexeus University Hospital in Barcelona, Spain, said the videos reveal for the first time that embryos exert “considerable force” and employ digging traction as they “invade” the uterine tissue, becoming completely integrated with it.
The findings, published in journal Science Advances, found crucial differences between how mouse and human embryos move in connecting to the uterus wall, the authors said.
An “ex vivo” platform they developed using an artificial uterine matrix made of gel and collagen which allows for implantation outside of a human uterus made the videos possible. The system could have a “significant impact” on efforts to counter infertility and help those who are unable to conceive naturally, they predicted.
Failure of the implantation process is the main reason behind the relatively low effectiveness of assisted reproductive technologies, such as in-vitro fertilization, in which embryos are conceived in a lab and then transferred to the womb. Implantation occurs in only 25% to 30% of transferred embryos — whether conceived in vivo or in-vitro — with embryo quality cited as the most significant feature affecting implantation.
“We’ve opened a window into a stage of development that was previously hidden,” the co-authors said in a statement to UPI. “After Day 5, when an embryo has 100 to 200 cells, it must implant, but until now, doctors couldn’t observe it again until an ultrasound weeks later.
“With our system, we can test culture conditions or compounds that might improve implantation.”
For example, the scientists say they have already developed a protein supplement that can be used in clinics to enhance implantation rates, available through their spin-off company Serabiotics and in collaboration with the Spanish pharmaceutical major Grifols.
“In short, this is a new tool for extending embryo observation and optimizing conditions for success,” they said.
The videos show a donated human embryo powerfully pulling on the uterine matrix and reshaping it as it goes, illustrating the importance of “optimal matrix displacement.”
Lead author Samuel Ojosnegros, principal investigator of IBEC’s Bioengineering for Reproductive Health Group, said the initial real-time look at a human embryo implanting itself was a profound experience for him.
“We had some experience making time-lapse movies of mouse embryos, but the first time we saw a human embryo implanting was truly astonishing,” he said. “Everything was different, the size, the shape, the behavior. They were stronger, more forceful, digging a hole into the matrix in a remarkably invasive way. Every detail felt unique.
“Watching it alive, in action, for the first time was absolutely mind-blowing.”
Embryo implantation is the “holy grail” of reproduction — and unlike in the animal world, in humans it can be a problematic process, resulting in about 1 in 6 people around the world having trouble making a baby, noted Dr. Mark Trolice, a professor at the University of Central Florida College of Medicine and founder/director of The IVF Center, a full-service reproductive medicine clinic in Orlando.
“Even though scientists have studied this for many years, they still do not fully understand how implantation works or what makes the uterus ready for an embryo,” he told UPI. “One big mystery is why a woman’s body can grow a baby made from sperm — which is a ‘foreign’ tissue — without rejecting it, as well as the ability to carry a donated egg.”
The new study, he said, “gives researchers a closer look at implantation. They used an ex vivo model, which means they studied the process outside the body. This let them watch how embryos interact with the uterine lining (called the endometrium) and measure the tiny pulling and pushing forces from both mouse embryos and donated human embryos.”
The videos showed for the first time that each species makes its own unique pattern of forces during implantation.
Trolice noted that while there are “some limits” to the Spanish study, “this work could lead to new ways of adjusting the uterine environment, which might help more embryos successfully implant.
“Before any treatment can be used, scientists will need to do human clinical trials. There are also important ethical and legal rules about using human tissues and embryos, which researchers must follow,” he added.