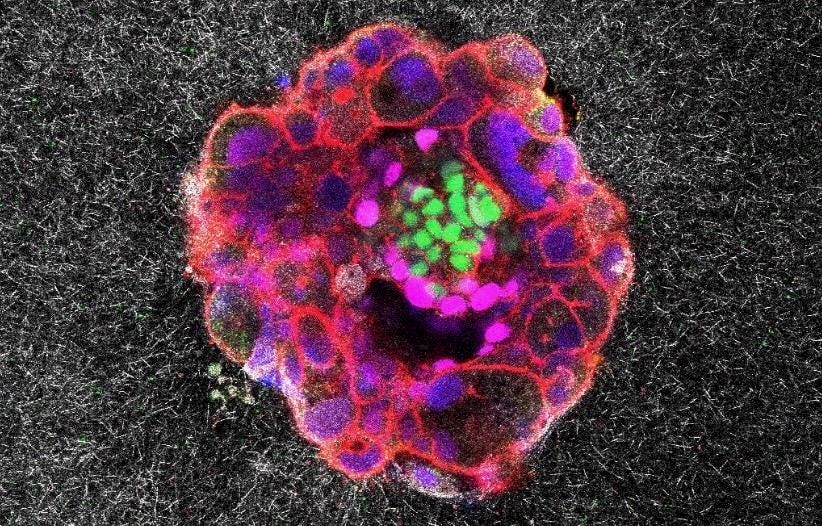
A confocal microscopy image of a nine-day-old human embryo, with specific proteins and cellular structures colored distinctively. Green relates to embryonic stem cells and magenta associates with early tissue formation. Blue marks the DNA in the nuclei and red which reveals the actin cytoskeleton.
Institute for Bioengineering of Catalonia (IBEC)
In the mood for an action film? Try the one starring a human embryo implanting. It just dropped, and it’s gripping.
Scientists at the Institute for Bioengineering of Catalonia in Barcelona have recorded footage of a key stage of human development in real time, and in 3D, for the first time. In doing so, they witnessed a surprisingly invasive event.
“We have observed that human embryos burrow into the uterus, exerting considerable force during the process,” Samuel Ojosnegros, principal investigator of the IBEC’s Bioengineering for Reproductive Health group, said in a statement. “These forces are necessary because the embryos must be able to invade the uterine tissue, becoming completely integrated with it.”
Ojosnegros is leader of a study published Friday in the journal Science Advances exploring how embryos overcome the maternal-tissue barrier. In their quest to implant, embryos release enzymes that break down the surrounding tissue. But while much implantation research focuses on the biochemical aspects of this complex and mysterious natural phenomenon, the IBEC team chose instead to examine the biomechanics.
Embryo, Meet Lab-Grown Womb
To investigate, they constructed an artificial uterine lining from a gel composed of collagen — a rigid protein abundant in fibrous uterine tissue — and other proteins needed for the embryo to develop. They then put dozens of human embryos left over from infertility treatments into the lab-made tissue matrix and used advanced high-resolution microscopy to capture them in action over time. Neither the matrix nor the embryo is naturally fluorescent, so the researchers had to rely on special techniques to visualize both.
(The first half of the video below shows the process of cell compaction in the embryo. The second part shows the embryo invading the artificial uterine lining.)
Past studies have observed tissue deformations that suggest cells apply force — when migrating and invading tumors, for example — and Ojosnegros and his team wanted to explore the hypothesis that this also happens in implantation.
“When we started placing embryos on fibrous matrixes, we noticed strong deformations in the material,” Ojosnegros said in an interview. “That immediately suggested that mechanics play a crucial role in implantation, so we decided to explore this dimension more deeply.”
Indeed, the footage of the cluster of cells meeting the uterus looks like a mini cosmic explosion. Many women experience abdominal pain and mild bleeding during implantation.
“We observe that the embryo pulls on the uterine matrix, moving and reorganizing it,” Amélie Godeau, a researcher in the Ojosnegros group and co-author of the study, said in a statement. “It also reacts to external force cues. We hypothesize that contractions occurring in vivo may influence embryo implantation.”
Barcelona’s Dexeus University Hospital, home to a large women’s health clinic, donated the embryos used in the study — all donor couples gave their signed informed consent and received information on the research project. Ayelet Lesman’s biomedical engineering lab at Tel Aviv University quantified the force of the embryos on the faux womb.
Eyes On Infertility Treatment
The team hopes their research could lead to breakthroughs in infertility treatment, as failed implantation is a leading cause.
“Nobody really knows how frequent implantation failure occurs because we have no way of diagnosing it,” Dr. Norbert Gleicher, an infertility specialist with New York’s Center for Human Reproduction, said in an interview. “This diagnosis, therefore, can only be assumed, and such assumptions are reached if an infertile woman had ‘X’ number of good quality embryos transferred in IVF without pregnancy and no evidence of any other cause of infertility.” Gleciher was not part of the team that conducted the study.
The researchers studied both mouse and human implantation to compare the mechanical differences. While the mouse embryo adheres to the uterine surface and then folds around it, essentially enveloping it, the human embryo is far more invasive. It completely penetrates the uterus and grows from the inside out by forming specialized tissues that connect to the mother’s blood vessels.
An ‘Important First Observation’
A number of reproductive scientists unaffiliated with the research out of IBEC have expressed excitement about it. Gleicher calls the new imagery an “important first observation,” but stresses that it only represents one piece of the complex equation that determines whether an implantation will succeed.
“Implantation is not simply a mechanical process,” he said. “It is a mechanical process, but at least in my opinion, it is first and foremost an immunological process” — one that determines whether the mother will tolerate the embryo or reject it.
Still, the new footage sheds further light on a primordial burst hidden deep within the body, one we owe our very existence to.
IBEC researchers studying the behavior of human embryos are, from left to right: Samuel Ojosnegros, Anna Seriola and Amélie Godeau.
Institute for Bioengineering of Catalonia (IBEC)