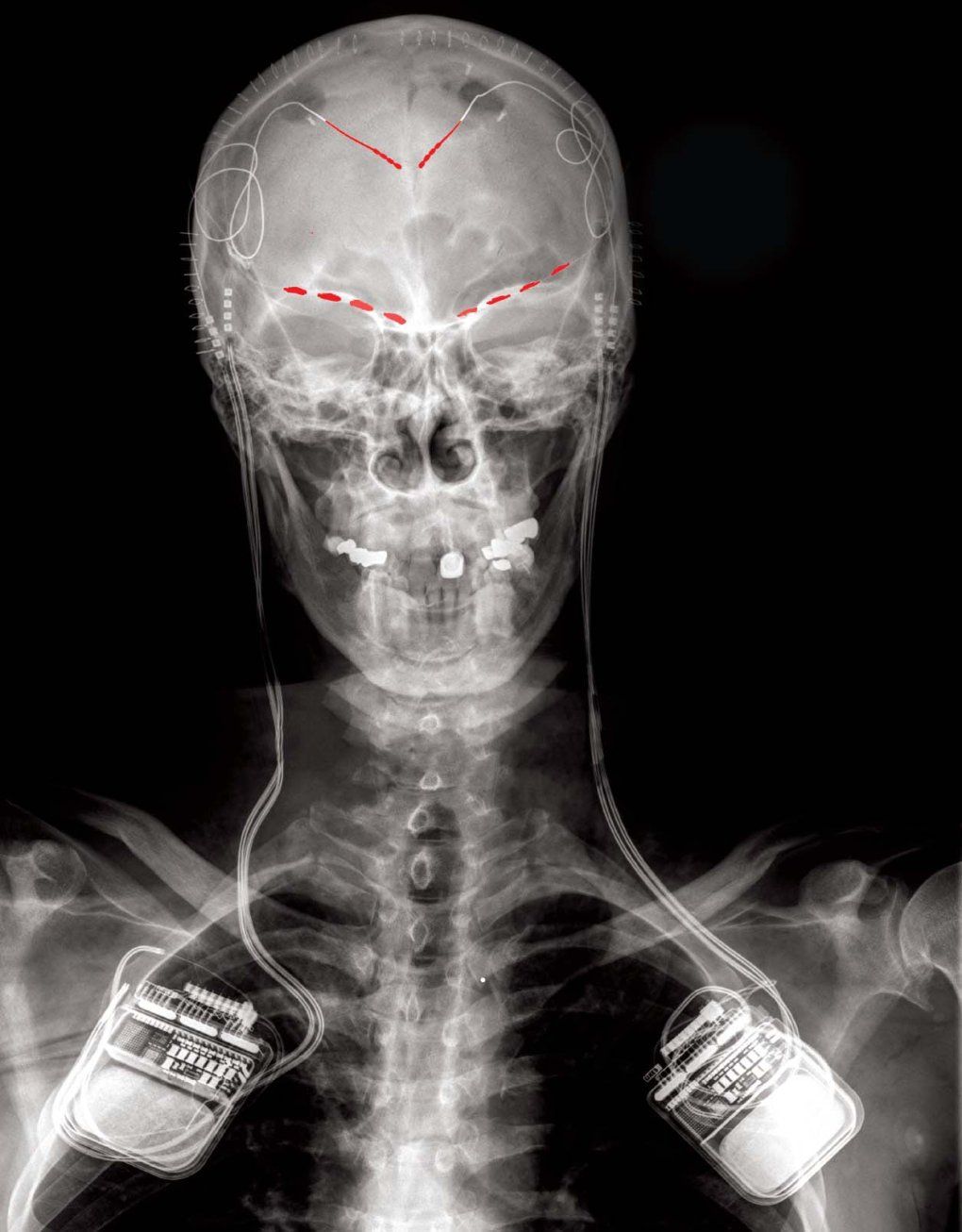
Electricity is the brain’s language. For a decade, National Institutes of Health (NIH) funding has enabled UC San Francisco physician-scientists to decipher this language and use deep brain stimulation to rewrite disease.
Deep brain stimulation delivers targeted electrical currents through tiny electrodes implanted in the brain. Like a cardiac pacemaker, these electrical pulses interrupt problematic brain activity — stopping tremors or pain signals before they take hold. While continuous deep brain stimulation has treated movement disorders like Parkinson’s disease for decades, the technology often lagged behind patients’ changing symptoms. And it hasn’t been reliably effective when tested against other conditions.
Over the past decade, UCSF Neurological Surgery Professors Philip Starr, MD, PhD, and Edward Chang, MD, have pioneered surgical and brain mapping techniques, ushering in personalized deep brain stimulation. With this breakthrough approach, developed in part at UCSF, electrical stimulation is only delivered when the device detects abnormal brain activity associated with symptoms — activity that is unique to each patient.
This advancement was made possible by funding from the NIH Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) initiative.
Here are four ways UCSF scientists are shaping the future of personalized deep brain stimulation to treat Parkinson’s disease and, perhaps one day, the most severe forms of chronic pain, depression, and obsessive-compulsive disorder.
Parkinson’s Disease
Shawn Connolly is one of a million Americans living with Parkinson’s disease, a progressive neurological disease that primarily affects movement. It can result in symptoms such as tremors, changes in walking, and slower movements.
When Connolly was diagnosed in 2015 at just 39 years old, the existing continuous deep brain stimulation technology fell short of providing relief from the ever-changing symptoms, which can vary from slowness and rigidity to waves of involuntary movement. Within five years, the former professional skateboarder was walking with a cane.
In 2021, Connolly joined a small clinical trial testing a self-adjusting deep brain stimulation approach developed by Starr, who co-directs the Movement Disorders and Neuromodulation Clinic and Simon Little, MBBS, PhD, assistant professor of neurology.
Their next-generation of deep-brain stimulation technology responded to Connolly’s Parkinson’s symptoms in real time. The approach used an algorithm that recognized brain signals that indicate a symptom was developing and directed the device to deliver just the right amount of electrical stimulation to prevent it. Unlike the traditional deep brain stimulation of the early 2000s — which was always on — this new version only provides electricity when needed.
In February, the Food and Drug Administration approve the use of two similar adaptive deep brain stimulation algorithms — one based on Little’s work — paving the way for the world’s first adaptive deep brain stimulation system for people with Parkinson’s.
“It’s definitely changed my life,” Connolly said in 2024. “I can just go through the whole day feeling good.”
Today, Starr and Chang’s insights into brain mapping are also aiding research into new, less invasive ways to obtain personalized deep brain stimulation’s results — without the surgery.
Connolly’s treatment was made possible by years of NIH support for Starr and Chang, who pioneered the use of electrocorticography, a type of monitoring that allows researchers to record signals from inside the brain. The technology allowed Starr to implant the first multi-site recording device in a Parkinson’s patient in 2013, setting the stage for the next generation of “as-needed” personalized deep brain stimulation. UCSF researchers continue to fine-tune the technology by developing new ways to measure Parkinson-related walking changes, one of its most disruptive symptoms, to improve deep brain stimulation.
Chronic Pain
Nearly a quarter of Americans live with chronic pain, or pain that drags on for at least three months. For many, their pain is resistant to any treatment.
In 1972, UCSF Neurological Surgery Professors John E. Adams, MD, and Yoshio Hosobuchi, MD, became the first in the U.S. to test continuous deep brain stimulation for chronic pain. Successive international teams attempted deep brain stimulation experiments for persistent pain for more than 50 years. Each team reproduced Adams’ and Hosobuchi’s fleeting results.
But scientists came to suspect that, over time, the brain got used to the “always on” current and, eventually, learned to outsmart it. Pain returned.
In 2023, Prasad Shirvalkar, MD, PhD, an associate professor of anesthesiology and neurological surgery, became the first to discover individual pain biomarkers, biological signals that can be detected and measured, by matching brain activity gathered via implanted electrodes with patient pain logs. He used artificial intelligence to predict when patients would be in pain based on their brain signals.
The discovery enabled clinical trials at UCSF of personalized deep brain stimulation systems that can sense when pain markers arise and then deliver targeted stimulation on demand — like a thermostat kicking in to cool a room that has gotten too warm. These initial studies could pave the way for larger trials, bringing this technology from lab to home.
Depression
Sarah’s depression was so bad when she met UCSF’s Edward Chang, MD, that she couldn’t see a way out.
“I was at the end of the line. I was severely depressed. I could not see myself continuing … if I could never move beyond this,” she told UCSF in 2021. “It was not a life worth living.”
Nearly 1 in 3 people diagnosed with major depressive disorder in the U.S. live with treatment-resistant depression like Sarah.
By 2018, Chang and colleagues had utilized advanced brain mapping techniques developed at UCSF to identify patterns of electrical brain activity that correlate with mood states and discover new brain regions that could be stimulated to alleviate someone’s depressed mood. In 2020, Chang’s team used this knowledge to provide Sarah with a personalized deep brain stimulation system that finally lifted her depression. Her success shows that personalized deep brain stimulation might, one day, be an effective treatment for others.
“In the early few months, the lessening of the depression was so abrupt, and I wasn’t sure if it would last,” she remembered. “But it has lasted. And I’ve come to realize that the device really augments the therapy and self-care I’ve learned while being a patient here at UCSF.”
The two approaches, deep brain stimulation and talk therapy, have helped bring her intrusive thoughts under control: “Those thoughts still come up, but it’s just … poof … the cycle stops.”
Today, UCSF Psychiatry Professor Andrew D. Krystal, MD, PhD runs a federally-funded trial studying deep brain stimulation and depression that, if successful, could help take the technology that saved Sarah’s life to millions more.
Obsessive-compulsive disorder and beyond
Today, NIH funding through the BRAIN Initiative and other programs has enabled UCSF to become one of only about a dozen hospitals that offer continuous deep brain stimulation as part of psychiatric care for intractable obsessive-compulsive disorder (OCD). OCD is marked by uncontrollable, recurring thoughts and repetitive, hard-to-resist behaviors, and it affects about 1 in 50 Americans. Currently, the Food and Drug Administration limits deep brain stimulation to only the most severe cases.
Andrew Moses Lee, MD, PhD, directs UCSF’s OCD Program. Lee is currently leading a small clinical trial to identify potential biomarkers of OCD symptoms in the brain and possible sites for personalized deep brain stimulation in the future. Together with Krystal, Lee is also leading a clinical trial to evaluate personalized deep brain stimulation for the condition.
Chang believes such treatments could help treat conditions like addiction, Tourette syndrome, and even Alzheimer’s disease, though each condition would have its own type of signature.
“Tailoring these treatments to the person’s neural signature is really the key that allows DBS to be effective across many conditions,” he said.

