Materials and methods
All reagents and solvents were obtained from commercial suppliers and used as supplied without further purification. Proton nuclear magnetic resonance spectra were determined on a Brucker Avance 500 spectrometer using tetramethylsilane as an internal standard in DMSO-d6 solution. The infrared spectra were determined on a FT-IR 370 infrared spectrometer with potassium bromide tablet pressing technology. The UV spectrum data were obtained using a TU-1810 UV Visible Spectrophotometer. The decomposition temperature was tested by a PerkinElmer TGA 4000 thermogravimetric analyzer. The whiteness and chromaticity index of cotton fiber were assessed using a CTPC whiteness meter. The microstructure of the sample was observed using a JSM-7610 F scanning electron microscope.
Synthesis of compounds 6a-f
2,4,6-Trichloro-1,3,5-triazine (1.28 mmol) and cold distilled water (5 mL) were placed in the reactor and subjected to an ice bath. The solution of 4,4’-diaminostilbene-2,2’-disulphonic acid (0.64 mmol), distilled water (20 mL), and saturated sodium carbonate was prepared, slowly dropped into the reactor within 30 min. Then the mixture continued to react for 2 h at 0–5℃. The reaction mixture was heated to 30℃, slowly added an aqueous solution (4 mL) containing 2-aminoethyl methacrylate (1.28 mmol), and small amount of antioxidant sodium sulfite. Then the reaction mixture was heated to 40–45℃, maintained the pH at 6–7 with saturated sodium carbonate, and continued to react for 6 h. The aqueous solution (5 mL) containing amino alcohol or amino acid (1.28 mmol) was dropped into the reaction mixture, raising the temperature to 90–95℃, maintaining the pH at 8–9 with saturated sodium carbonate, and continuing the reaction for 8 h. The three-step reaction process was tracked using thin-layer chromatography, with isopropanol: acetonitrile: ammonia solution = 5:2:3 as the developing agent (V/V). The reaction mixture was cooled to room temperature, and the pH was adjusted to 3–4 with dilute hydrochloric acid (10%). An appropriate amount of sodium chloride was added, stirred, and filtered. The filter cake was washed successively with ice water and ethanol to obtain aminoethyl methacrylate functionalized stilbene FWAs 6a-f, light yellow solid with yield of 85–93%.
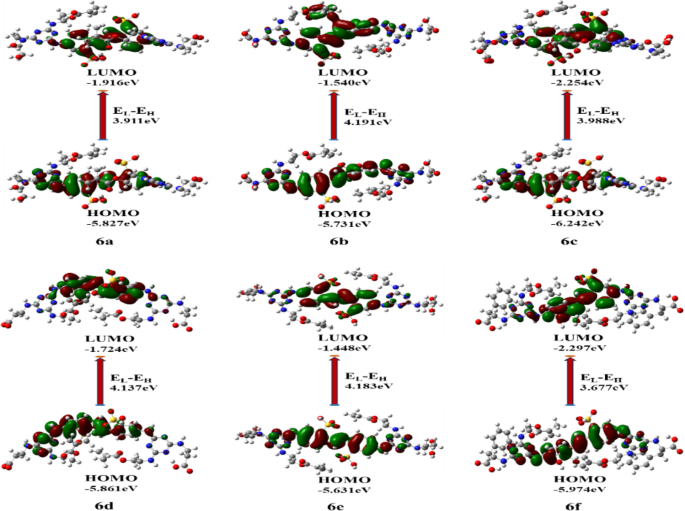
Synthesis of 6,6’-(ethene-1,2-diyl)bis(3-((4-(bis(2-hydroxyethyl)amino)-6-((2-(methacryloyloxy)ethyl)amino)-1,3,5-triazin-2-yl)amino)benzenesulfonic acid) (6a)
Yield 87%; Light yellow solid powder; 1H NMR (500 MHz, DMSO-d6, δ): 10.35 (2 H, NH), 8.52 (2 H, NH), 8.04 (2 H, PhH), 7.62 (4 H, PhH), 7.47 (2 H, CH = CH), 6.07 (2 H, =CH2), 5.70 (2 H, =CH2), 4.25 (4 H, OH), 3.75 (20 H, CH2), 3.00 (4 H, CH2), 1.88 (6 H, CH3); IR (KBr, cm− 1): 3346 (OH and NH), 2952, 2886 (CH3 and CH2), 1718 (C = O), 1625, 1538 (C = C), 1415 (C = N), 1173 (S = O), 1020 (C‒O‒C).
Synthesis of 2,2’-((((ethene-1,2-diylbis(3-sulfo-4,1-phenylene))bis(azanediyl))bis(6-((2-(methacryloyloxy)ethyl)amino)-1,3,5-triazine-4,2-diyl))bis(azanediyl))diacetic acid (6b)
Yield 85%; Light yellow solid powder; 1H NMR (500 MHz, DMSO-d6, δ): 10.12 (2 H, NH), 8.30 (2 H, NH), 8.01 (2 H, PhH), 7.58 (4 H, PhH), 7.55 (2 H, CH = CH), 6.06 (2 H, =CH2), 5.66 (2 H, =CH2), 4.29 (2 H, NH), 3.82 (8 H, CH2), 3.71 (4 H, CH2), 1.86 (6 H, CH3); IR (KBr, cm− 1): 3397 (OH and NH), 3086, 2941(CH3 and CH2), 1749 (C = O), 1623, 1588 (C = C), 1485 (C = N), 1181 (S = O), 1082 (C‒O‒C).
Synthesis of 2,2’,2’’,2’’’-((((ethene-1,2-diylbis(3-sulfo-4,1-phenylene))bis(azanediyl))bis(6-((2-(methacryloyloxy)ethyl)amino)-1,3,5-triazine-4,2-diyl))bis(azanetriyl))tetraacetic acid (6c)
Yield 86%; Light yellow solid powder; 1H NMR(500 MHz, DMSO-d6, δ): 10.57 (2 H, NH), 8.04 (2 H, NH), 7.80 (2 H, PhH), 7.66 (4 H, PhH), 7.61 (2 H, CH = CH), 6.07 (2 H, =CH2), 5.70 (2 H, =CH2), 4.21 (4 H, CH2), 3.54 (8 H, CH2), 3.39 (4 H, CH2), 1.88 (6 H, CH3); IR (KBr, cm− 1): 3328 (OH and NH), 3085, 2954 (CH3 and CH2), 1717 (C = O), 1630, 1591 (C = C), 1482 (C = N), 1168 (S = O), 1084 (C‒O‒C).
Synthesis of 3,3’-((((ethene-1,2-diylbis(3-sulfo-4,1-phenylene))bis(azanediyl))bis(6-((2-(methacryloyloxy)ethyl)amino)-1,3,5-triazine-4,2-diyl))bis(azanediyl))dipropionic acid (6d)
Yield 93%; Light yellow solid powder; 1H NMR (500 MHz, DMSO-d6, δ): 10.23 (2 H, NH), 8.38 (2 H, NH), 8.01 (4 H, PhH), 7.78 (2 H, PhH), 7.65 (2 H, CH = CH), 7.58 (2 H, NH), 6.06 (2 H, =CH2), 5.68 (2 H, =CH2), 4.24 (4 H, CH2), 3.70 (4 H, CH2), 3.56 (4 H, CH2), 2.61 (4 H, CH2), 1.86 (6 H, CH3); IR (KBr, cm− 1): 3399 (OH and NH), 3086, 2958 (CH3 and CH2), 1705 (C = O), 1621, 1584 (C = C), 1489 (C = N), 1175 (S = O), 1020 (C‒O‒C).
Synthesis of 4,4’-((((ethene-1,2-diylbis(3-sulfo-4,1-phenylene))bis(azanediyl))bis(6-((2-(methacryloyloxy)ethyl)amino)-1,3,5-triazine-4,2-diyl))bis(azanediyl))dibutyricacid (6e)
Yield 91%; Light yellow solid powder; 1H NMR (500 MHz, DMSO-d6, δ): 10.19 (2 H, NH), 8.34 (2 H, NH), 8.01 (4 H, PhH), 7.79 (2 H, PhH), 7.65 (2 H, CH = CH), 7.58 (2 H, NH), 6.06 (2 H, =CH2), 5.67 (2 H, =CH2), 4.29 (4 H, CH2), 3.70 (4 H, CH2), 3.56 (4 H, CH2), 2.31 (4 H, CH2), 1.86 (6 H, CH3), 1.76 (4 H, CH2); IR (KBr, cm− 1): 3403 (OH and NH), 3121, 2956 (CH3 and CH2), 1701 (C = O), 1621, 1571 (C = C), 1489 (C = N), 1175 (S = O), 1025 (C‒O‒C).
Synthesis of 2,2’-((((ethene-1,2-diylbis(3-sulfo-4,1-phenylene))bis(azanediyl))bis(6-((2-(methacryloyloxy)ethyl)amino)-1,3,5-triazine-4,2-diyl))bis(azanediyl))bis(3-phenylpropanoic acid) (
6f
)
Yield 86%; Light yellow solid powder; 1H NMR (500 MHz, DMSO-d6, δ): 10.19 (2 H, NH), 8.81 (2 H, NH), 8.04 (2 H, PhH), 7.58 (4 H, PhH), 7.30 (10 H, PhH), 7.22 (2 H, CH = CH), 6.06 (2 H, =CH2), 5.66 (2 H, =CH2), 4.77 (2 H, NH), 4.29 (4 H, CH2), 3.24 (4 H, CH2), 3.03 (4 H, CH2), 1.86 (6 H, CH3); IR (KBr, cm− 1): 3397 (OH and NH), 3084, 2958 (CH3 and CH2), 1716 (C = O), 1623, 1590 (C = C), 1487 (C = N), 1177 (S = O), 1020 (C‒O‒C).
Cotton fiber dyeing
Dye solutions with concentrations of 0.10%, 0.20%, 0.30%, 0.40%, 0.50%, 0.60%, and 0.70% of compounds 6a-f were prepared and the bath ratio was 1:50. Cotton fibers were placed in a dyeing bucket and dyed at 20℃, and the dyeing solution was heated by a rate of 2℃/min until the temperature reached 50℃, and then continued to dye for 15 min at this temperature. The stained sample was taken out, washed three times with cold distilled water, dried it naturally at room temperature, and tested for whiteness and chromaticity index.
Whiteness and chromaticity index testing
The whiteness value (CIE) and chromaticity index of untreated and dyed pure cotton fiber samples were investigated using a multifunctional whiteness meter with a D65 light source and a viewing angle of 10°. Each sample was tested three times at different locations and the average value was recorded.