The ESMO Asia 2025 Congress took place from December 5–7, 2025, at the Suntec Singapore Convention & Exhibition Centre in Singapore. The three-day meeting brought together oncology professionals from across the Asia-Pacific region to review the latest advances in cancer research and clinical practice.
The scientific program featured plenary lectures, expert sessions, and multidisciplinary tumor boards that highlighted progress in systemic therapies, immuno-oncology, precision medicine, and supportive care. Attendees also engaged in discussions on evolving treatment standards and regional challenges in oncology.
20 Posts Not to Miss from ESMO Asia 2025
In this article, we selected 20 key posts you shouldn’t miss, capturing the most impactful insights, research updates, and expert perspectives shared throughout ESMO Asia 2025.
Herbert Loong, MBBS, FASCO:
“Kicking off activities at ESMOAsia25 with a short talk on AI driven Biomarkers and what are the potentials and implications of these in drug development and oncology clinical care. A shoutout to the just released EBAI guidance document: check it out here: https://doi.org/10.1016/j.annonc.2025.11.009 Ben Westphalen and many others”
Melvin LK CHUA:
“ESMO Asia 25 Packed room for Radiation Oncology session! Such a strong line up of speakers!”

Teresa Amaral MD:
“The ESMOAsia25 ongoing patient engagement summit
Excellent discussion with patient advocates, listening and learning about the challenges patients and caregivers are facing this part of the globe .
A proud moment to see the ESMO – European Society for Medical Oncology ISF EISF project on Health Equity & hashtag#Inclusive Research PG.
You can read more about this here https://lnkd.in/d4c43_2p
Tomorrow I will talk about “Advancing diversity in clinical trials: how Europe is meeting the challenge” and I am looking forward to a lively discussion.
Thank you to the ESMO Public Policy for the invitation.”

Angela Lamarca:
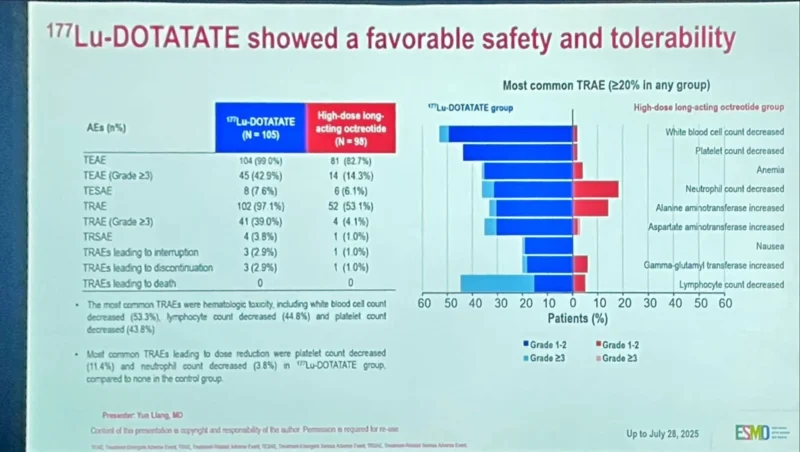
“Very interesting discussion on toxicity Higher (x2-3 times ) haemato-toxicity (grade 1/2) in China (LUMINET-1 1) vs Western (NETTER-1 2) Should dose be the same? Maybe not (I agree Dr Llang) Stephen Chan (as ussual)”
Hongcheng Zhu:
“Super engaged ESMOYOC session of ESMOAsia25 with Vesalius talk about Research, Education, & Collaboration for YoungOncologists in Asia-Pacific, fantastic discussion with our amazing international experts.”

Herbert Loong, MBBS, FASCO:
“Amazing team at the educational session for lung cancer during ESMOAsia25! ”

Jarushka Naidoo:
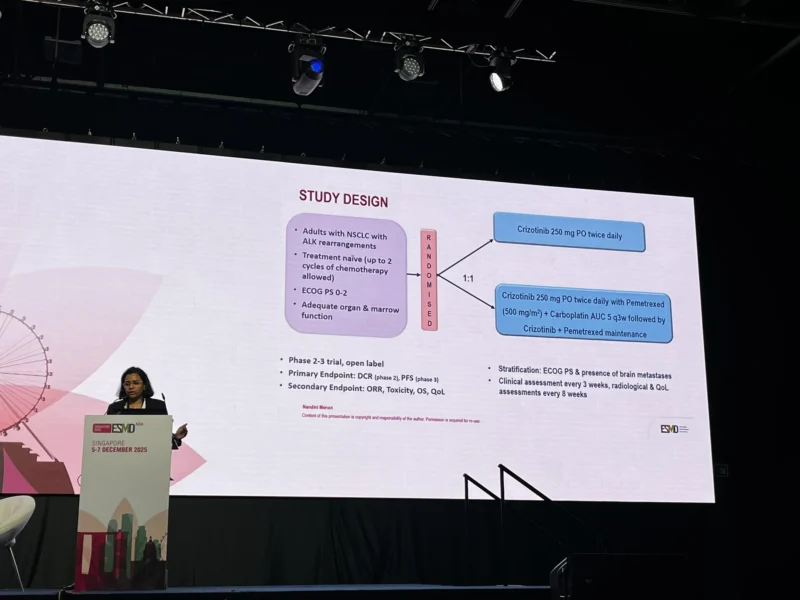
“ESMOAsia2025 Investigator-initiated 1L Ph II/III trial Crizotinib+Chemo v Criz in ALK+ NSCLC Tata Memorial – study stopped early – DCR 55% v 75% in favour of Criz – 11SAEs 8 deaths- sepsis main tox A cautionary tale- more is not always more.”
Angela Lamarca:
“Here we are, a few of the GI people at ESMOAsia25 It’s always a pleasure working with you all Great co-chairing of the #ESMOAsia conference, Fantastic track chairs, Lorenza Rimassa and Dr Ikeda To many more of these…”

Foo Chuan Jie:
“Challenges and opportunities of oncology early-phase drug development programmes in Asia. ESMOAsia25 day2. Exciting discussions with Prof. Toshio Shimizu and Adj. Prof. Voon Pei Jye! for Phase 1!”

Jarushka Naidoo:
“ESMOAsia2025 Lung Orals MARIPOSA: Asian subgroup analysis – HR OS 0.74 – majority of MARIPOSA made up of asian subset – similar tox signal, & crossing of curves at 9m.”

Angela Lamarca:
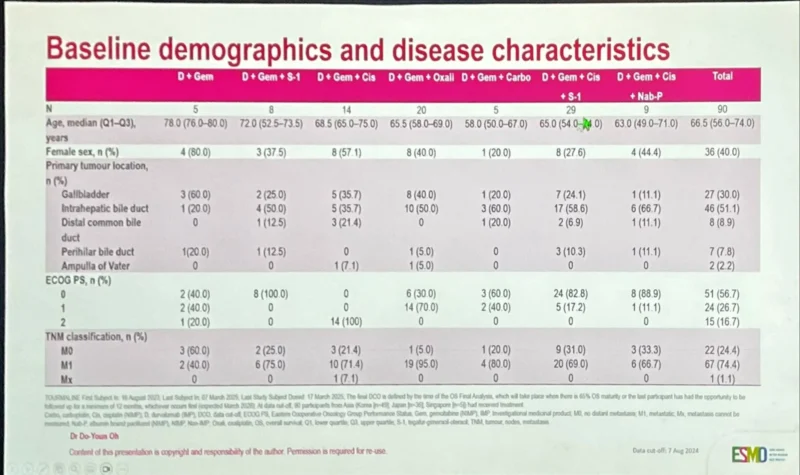
“Proffered Paper in BTC at ESMOAsia25 TOURMALINE (DurvaChemo nonTOPAZ) in #Asia (90 pts) iCCA51%; GBC30%; PS2 16.7% 52% G3-4 AEs – haem/cholangitis; 13% anyG imAE; 6.7% IRR 26.7% ORR – best CisGem (PS2; ORR 50%) Confirms data in Asia Doublet (vs mono) chemo best.”
Yuichiro Kikawa, MD, PhD:
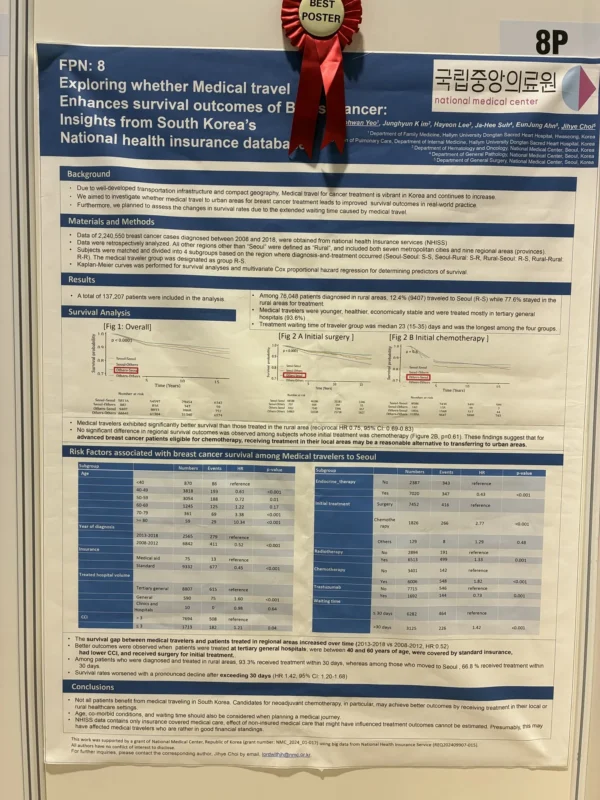
“Korea NHIS breast cancer: ~12% of rural pts traveled to Seoul. Travelers were younger/healthier, treated at tertiary centers, but had longer waits. Survival improved overall vs rural care (HR ~0.75); no clear benefit when initial tx was chemo. Avoid >30-day delays.”
Deborah Mukherji:
“Excellent discussion in the GU Mini Oral session this morning ESMOAsia25 thoughtfully putting new data into clinical context and highlighting what it means for real-world practice across Asia. Great insights from Dr Senthil Rajappa”

Jordi Remon:
“Uncommon / PACC EGFR mut NSCLC are 10% of all EGFRm.Afatinib is the unique drug approved in this subset. Enozertinib (ORIC-114):promising activity in PACC EGFRm in 3L with intracranial activity Other “competitors” : furmonertinib, zipalertinib, amivantamab+lazertinib”
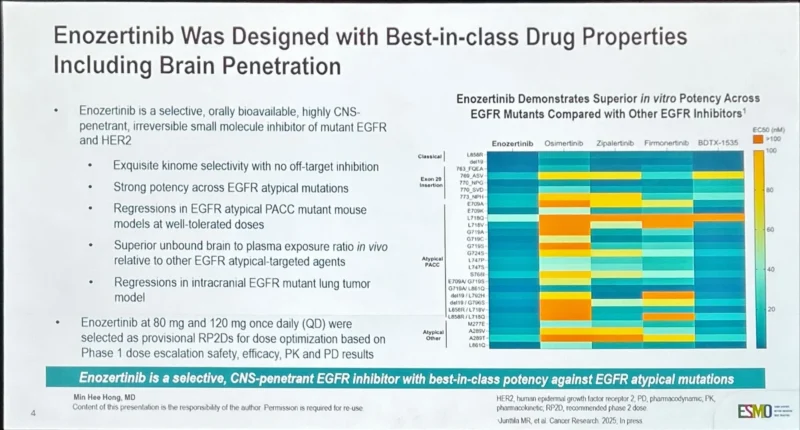
Herdee Luna:
“Congratulations Annie Wong and the whole ESMO Asia LGP family who contributed to this abstract on APAC Availability, OPC, and Accessibility of AntiNeoplastic Medicines!”

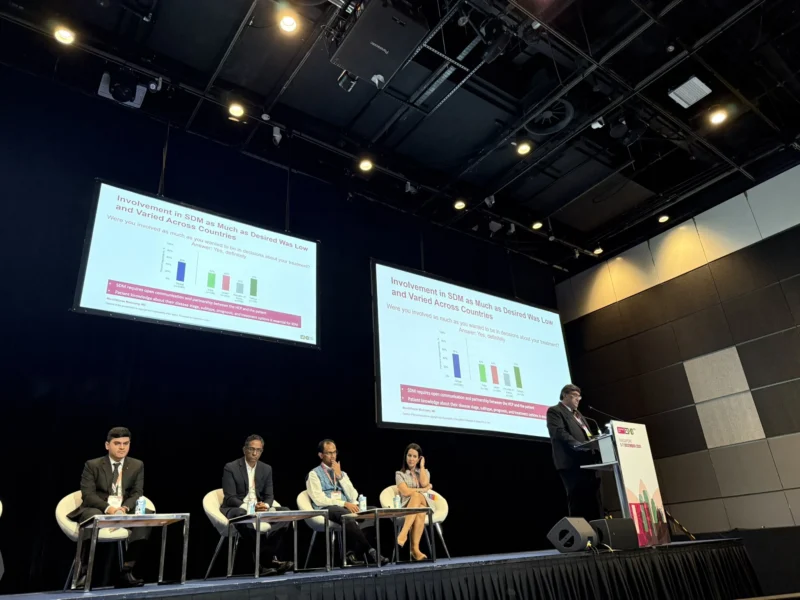
Deborah Mukherji:
“Patient-reported data are essential to understanding real gaps in cancer care. IKCC GPS 2025 shows that SDM remains limited across Asia and up to 92% of kidney cancer patients face treatment barriers. Listening to patients must guide action.”
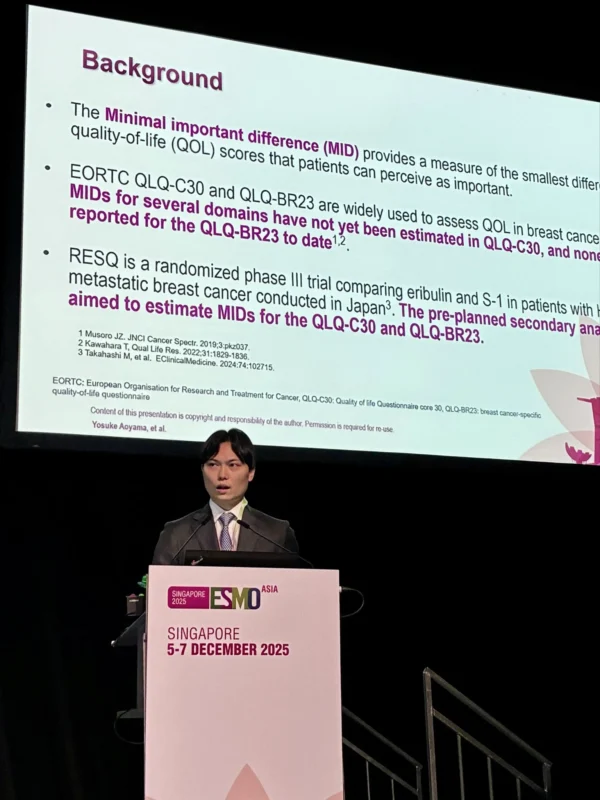
Yuichiro Kikawa, MD, PhD:
“Proud to share our study from Japan’s phase 3 RESQ trial estimating MIDs for EORTC QLQ-C30/BR23 in HER2− metastatic BC. First MID estimates for QLQ-BR23 Expanded QLQ-C30 benchmarks Excellent presentation by Dr. Aoyama—clear and impactful.”
Lorenza Rimassa:
“Meet the expert: ESMO GI Oncology Journal in Asia. Su Pin Choo moderates Florian Lordick discussing gastric cancer and claudin 18.2 in Asia at ESMOAsia25 in Singapore”

Long Nguyen:
“Glad to see an increasing representation of Vietnamese young oncologist at major international oncology meetings! ESMOASIA25”

Foo Chuan Jie:
“Challenges and opportunities of oncology early-phase drug development programmes in Asia. ESMOAsia25 day2. Exciting discussions with Prof. Toshio Shimizu and Adj. Prof. Voon Pei Jye! for Phase 1!”

More post about ESMO Asia 2025 on OncoDaily.
