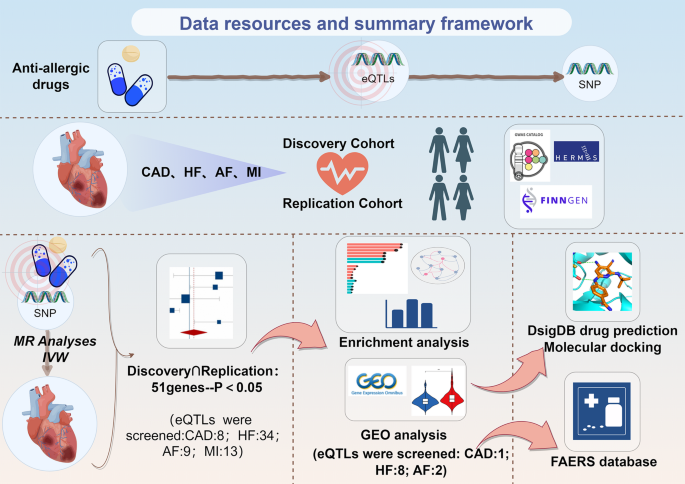
A comprehensive MR analysis was performed on 139 eQTLs linked to anti-allergic drugs within discovery cohorts of 122,733 CAD patients, 68,157 HF patients, 395,795 MI patients, and 60,620 AF patients. Subsequently, these eQTLs were validated in replication cohorts including 42,096 CAD patients, 47,309 HF patients, 28,546 MI patients, and 55,853 AF patients. The analysis identified 51 eQTLs associated with anti-allergic drugs that are linked to CVDs. Specifically, 8 eQTLs were related to CAD, 34 to HF, 9 to AF, and 13 to MI. Enrichment analysis emphasized the importance of pathways such as lipid and atherosclerosis, neutrophil extracellular trap formation, and the thyroid hormone signaling pathway. Furthermore, 11 eQTLs were identified with stronger associations to CVDs, including KAT2A, HPGD, GLS, AMD1, TSHR, CLOCK, POLB, ITGB2, TG, BAZ2B, and CYP2C8.The FAERS database enabled analysis of cardiovascular adverse events associated with prednisone (an anti-allergic drug), identifying ITGB2 and TG as high-risk targets in HF patients.
Biological insights
Numerous studies have reported the potential impact of anti-allergic drugs on CVDs. For example, cohort and retrospective studies have found that some antihistamines, such as second-generation antihistamines and β2 receptor agonists, are associated with the development of cardiovascular events like HF and arrhythmias23. However, a study has shown that the target TRPV2, inhibited by the anti-allergic drug tranilast, may be a novel therapeutic target for HF5. Ingelsson E and colleagues conducted a cohort study indicating that montelukast may have a potential role in the secondary prevention of recurrent CVDs24. Similarly, Göbel et al. reported that zafirlukast could confer cardiovascular benefits by inhibiting soluble epoxide hydrolase (sEH) and activating peroxisome proliferator-activated receptors (PPARs)25.
In the study of 11 eQTLs linked to anti-allergic drugs, it was observed that several drug targets influence the development of CVDs through pathways related to lipid metabolism and signal transduction. This finding may shed light on the biological mechanisms underlying the causal relationship between anti-allergic drugs and CVDs risk. This research highlights a negative correlation between KAT2A and CAD. KAT2A (the drug target identified by database screening is zafirlukast)may improve heart function by modulating histone acetylation and the expression of antioxidant genes through the thyroid hormone signaling pathway and histone modifying activity26,27 Additionally, zafirlukast affects other targets such as BAZ2B and POLB. BAZ2B is a chromatin remodeling factor involved in cardiovascular immune regulation28,29while POLB plays a crucial role in DNA repair, which is vital for maintaining cardiovascular cell genome stability30. TSHR, a G-protein coupled receptor located on thyroid cells, regulates thyroid function. TSHR (the drug target identified by database screening is loratadine)may enhance thyroid hormone synthesis and secretion via the cAMP signaling pathway and cellular response to peptide processes. However, TSHR may also induce mitochondrial oxidative damage in endothelial cells, potentially affecting heart function31,32. We screened the database and identified prednisone as a potential targeted drug for ITGB2, an integrin beta-2 subunit involved in inflammation. ITGB2,has the potential to modulate immune and inflammatory signaling in myocardial cells by impacting pathways such as neutrophil extracellular trap formation and membrane raft composition33,34. This interaction could impact heart function and adaptability, potentially leading to HF. TG, a precursor in the synthesis of thyroid hormone, is also targeted by prednisone potentially impacting cardiovascular health through modulation of thyroid hormone levels35. Finally, montelukast targets CYP2C8, a key enzyme involved in drug metabolism, potentially modulating lipid levels and influencing drug metabolism through its impact on lipid and atherosclerosis pathways36,37.
Pharmacovigilance
The present study identifies two drug targets, ITGB2 and TG, which exhibit a causal relationship with HF and demonstrate high expression levels in HF. These targets are linked to prednisone, a corticosteroid with anti-inflammatory and immunosuppressive properties. Prednisone is widely used to manage various inflammatory conditions, but its multi-target effects may result in complex drug interactions, potentially increasing the risk of cardiovascular adverse events when combined with HF medications. Firstly, the research suggests that prednisone might exacerbate heart function by affecting pathways involved in neutrophil extracellular trap formation, membrane raft components, and myocardial cell metabolism. Additionally, prednisone could activate the Atrogin-1 pathway, leading to myocardial cell atrophy and compromised cardiac contractility38. Secondly, the concurrent use of prednisone with HF medications could lead to drug interactions. For example, co-administration of prednisone with digoxin may alter digoxin’s pharmacokinetics, increasing its blood concentration39. Prednisone may also argument the cardiovascular system’s responsiveness to catecholamines, thereby elevating the risk of HF exacerbation. Therefore, HF patients should be closely monitored for potential drug interactions and adverse effects when using prednisone in combination with other HF treatments.
Strengths and limitations
Firstly, the association between anti-allergic drugs and CVD outcomes was examined and validated within two independent cohorts, thereby enhancing the reliability of the results. Secondly, multiple analytical methods were employed to test these associations, with the consistency across various datasets further supporting the findings. Thirdly, by utilizing FAERS database information in conjunction with drug labels, the credibility of the conclusions was strengthened. However, some limitations should be considered when interpreting the findings. Firstly, the analysis primarily focused on European populations, and further validation is required to determine if these findings are generalizable to other populations, as genetic variants in drug-metabolizing genes (e.g., CYP2C19) show ethnic-specific frequencies that may alter drug efficacy. Additionally, the study did not stratify prednisone exposure by dose or duration, limiting the ability to distinguish between short-term (low-risk) and chronic (high-risk) cardiovascular effects—FAERS data aggregating events across all exposure scenarios may overstate clinical concerns for routine short-term use. Although sensitivity analyses (MR-Egger, weighted median) were performed to detect pleiotropy, residual effects from unmeasured confounders cannot be ruled out, and eQTLs used as IVs may not fully capture anti-allergic drugs’ pharmacodynamic effects as they reflect genetic variation rather than direct drug-target interactions. The linkage between genes (e.g., KAT2A) and anti-allergic drugs (e.g., zafirlukast), inferred from databases (DGIdb, DrugBank), lacks direct human evidence, introducing mechanistic uncertainties. Future research should delve into different doses and treatment regimens for specific patient subgroups, validate findings in multi-ethnic cohorts, explore specific interactions between more anti-allergic and cardiovascular drugs, and strengthen mechanistic links through large-scale prospective cohort studies and experimental validation.