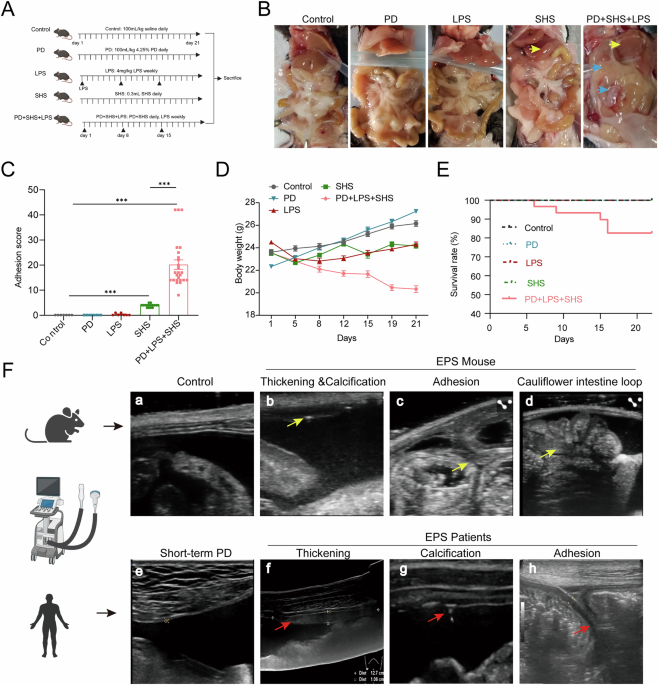
Establishing a mouse model of EPS with rapid intra-abdominal adhesions
We aimed to develop a novel and more efficient mouse model of encapsulating peritoneal sclerosis (EPS), closely mimicking the condition observed in human patients. To achieve this, we combined several high-risk factors commonly associated with peritoneal dialysis-related EPS, including a 4.25% peritoneal dialysis solution (PD), a surgical hygiene solution (SHS) consisting of chlorhexidine gluconate and ethanol, and lipopolysaccharide (LPS) to replicate episodic peritonitis (Fig. 1A). While traditional models using SHS alone require up to 8 weeks for EPS formation [13], the addition of PD and LPS successfully accelerated the process, enabling significant intra-abdominal adhesion formation by the 3rd week.
A Schematic of the experimental regimen for establishing the mouse model of EPS. B Gross macroscopic examination of the abdominal cavity. Control mice displayed smooth, well-expanded mesenteries and sharp liver edges; PD group and LPS group showed no observable changes; SHS-treated mice showed blunted liver edges (yellow arrow), but little interorgan adhesions. In the PD + LPS + SHS group, there were extensive adhesions between abdominal organs (blue arrow) and thickened liver margins caused by surface fibrosis. C Quantification of adhesion scores across Control group (n = 7), PD group (n = 6), LPS group (n = 7), SHS group (n = 16), and PD + SHS + LPS group (n = 25). D Dynamic body weight changes in mice over the course of the study. E Kaplan–Meier survival curve showing survival rates in each group. EPS mice obtained a survival rate of 83.3% (25/30) and a success rate of 100% (25/25). F Ultrasonographic comparison highlights the anatomical similarities between the murine EPS model and the human condition. a–d Ultrasound imaging of control mice revealed a smooth parietal peritoneum and normal gastrointestinal motility (a). In contrast, the EPS mice showed peritoneal thickening and calcification (b, arrows), adhesions between the parietal peritoneum and intestinal loops (c, arrows), and a characteristic “cauliflower-like” central clumping of small bowel loops (d). Supplementary Video 1 provides dynamic ultrasonography of the abdominal cavity in both control and EPS mice. e–h In human patients, control subjects demonstrated a smooth peritoneal surface (e), while EPS patients exhibited peritoneal thickening (f, arrows), calcification (g, arrows), and significant adhesions between the parietal peritoneum and intestinal loops (h, arrows). ***p < 0.001 by Student’s t test or ANOVA test. Fig. 1A, and the ultrasound, human, mouse drawing elements in Fig. 1F were created in BioRender. Sun, J. (2025) https://BioRender.com/vbajaol.
Macroscopic evaluation revealed distinct differences between the experimental groups. Control mice showed smooth hepatic edges and well-expanded mesenteries with no visible adhesions (Fig. 1B). In contrast, the PD + LPS + SHS group exhibited prominent signs of adhesion, including thickened liver margins with fibrous deposits (Fig. 1B, yellow arrow) and extensive adhesions between the abdominal organs, particularly between the liver, intestines, and parietal peritoneum (Fig. 1B, blue arrow). Additionally, these mice displayed severe mesentery contraction and intestinal dilation, with some cases forming an abdominal “cocoon” structure, indicative of advanced EPS pathology (Supplemental Fig. 1). The adhesion scores of the PD + LPS + SHS group were significantly higher than those of the single control groups, demonstrating the robustness of the model in replicating EPS (Fig. 1C).
To assess the physiological impact of EPS, we monitored body weight and survival rates. Mice in the PD + LPS + SHS group showed significant weight loss compared to other groups, suggesting a progression toward malnutrition or cachexia, a common complication in EPS (Fig. 1D). Despite the severity of the disease, the survival rate in this group remained relatively high at 83.3%, allowing us to reliably conduct further analyses (Fig. 1E).
Since invasive exploration of the abdominal cavity is impractical in clinical settings, we employed ultrasonography to non-invasively assess the structural changes in the peritoneum, as commonly done in patients20. Comparing to the control group (Fig. 1F-a, Supplementary Video 1), ultrasonography of EPS mice revealed key pathological features, including peritoneal thickening, calcification, and characteristic intestinal dilation (Figs. 1F-b and 1F-c), resembling the “concertina-like” appearance often observed in EPS patients. The mouse model also demonstrated strong adhesions between the parietal peritoneum and intestinal loops, which were confirmed by static and dynamic imaging (Fig. 1F-d, Supplementary Video 2). To validate the clinical relevance of our model, we compared these findings with ultrasonographic features from human EPS patients. The human subjects exhibited similar pathological traits, such as peritoneal thickening, calcification, and organ adhesions (Figs. 1F-f to 1F-h). These similarities between the mouse model and human disease underscore the utility of this model for studying EPS pathogenesis and evaluating potential therapeutic interventions. In summary, the combination of PD, LPS, and SHS successfully accelerated the development of EPS in mice, with key anatomical and pathological features closely mimicking human EPS. Given its efficiency and reproducibility, this model will be used for further studies aimed at understanding the mechanisms of EPS and testing potential therapeutic strategies.
EPS mouse model exhibits inflammation, fibrosis, and increased vascular density in peritoneum
To further characterize the pathological changes in our EPS mouse model, we performed detailed histopathological analyses of the peritoneum. As shown in Fig. 2A, cross-sectional images of the abdominal cavity revealed widespread and diffuse adhesions throughout the peritoneum in EPS mice. These adhesions formed dense, clot-like structures that encompassed multiple abdominal organs, closely resembling advanced EPS pathology observed in humans. Histological staining provided insight into the structural and cellular changes associated with EPS. Hematoxylin and eosin (H&E) staining indicated substantial thickening of both the parietal and visceral peritoneum in the EPS group, with marked infiltration of inflammatory cells (Fig. 2B, C). This infiltration signifies a heightened inflammatory response within the peritoneal tissues, which is a key feature of EPS progression. In addition, Masson’s trichrome staining highlighted extensive fibrotic deposition, further confirming that fibrosis is a central component of EPS pathology (Fig. 2B, C). Quantitative analysis of peritoneal thickness revealed a significant increase in EPS mice compared to controls, as depicted in Fig. 2G, H, reflecting the overall fibrotic burden in the disease. To explore the molecular drivers of this fibrotic response, we conducted immunohistochemical analyses, which demonstrated the upregulation of extracellular matrix (ECM) markers, including collagen type I alpha 1 (COL1A1), fibronectin (FN) and α-smooth muscle actin (α-SMA), in the peritoneum of EPS mice (Fig. 2D, Supplemental Fig. 2A). These markers are indicative of active fibroblast proliferation and matrix remodeling, key processes in tissue fibrosis.
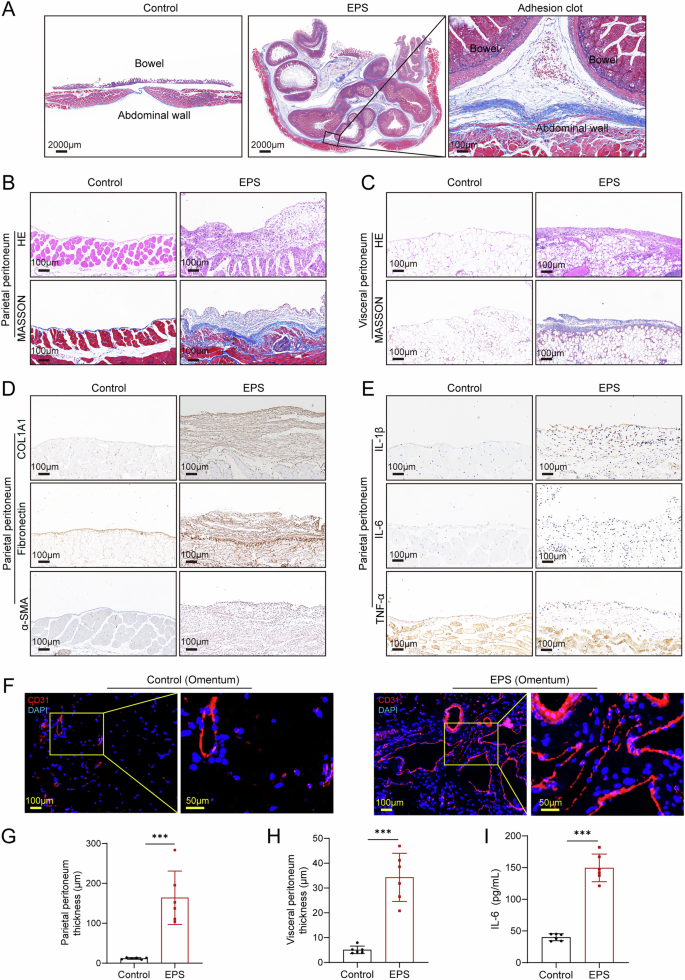
A Cross-sectional images of the abdominal cavity in EPS mice, showing widespread, diffuse adhesions forming clot-like structures throughout the peritoneal cavity. Hematoxylin and eosin (HE) and Masson’s trichrome staining of the parietal (B) and visceral (C) peritoneum, demonstrating significant peritoneal thickening and fibrotic deposition in EPS mice. D Immunohistochemical analysis of the parietal peritoneum showing increased expression of extracellular matrix (ECM) markers, including COL1A1, fibronectin (FN), and α-smooth muscle actin (α-SMA), indicative of active fibrosis. E Immunohistochemical analysis of inflammatory markers IL-1β, IL-6, and TNF-α in the parietal peritoneum, showing significant upregulation of these cytokines in EPS mice, highlighting the inflammatory component of the disease. F Immunofluorescent staining of CD31 in the visceral peritoneum (omentum) showing denser angiogenesis in EPS mice compared to controls. Quantification of peritoneal thickness in the parietal (G) and visceral (H) peritoneum, revealing significant thickening in EPS mice (n = 6 per group). I ELISA analysis of peritoneal lavage fluid, confirming an increase in IL-6 levels in EPS mice compared to controls (n = 6 per group). Data are presented as mean ± SEM.*** p < 0.001, two-tailed Student’s t-test.
Inflammation is known to play a critical role in EPS development21,22. In consistent with these observations, our immunohistochemical analysis showed significant upregulation of pro-inflammatory cytokines, including interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α), within the peritoneal tissues of EPS mice (Fig. 2E, Supplemental Fig. 2B). Furthermore, enzyme-linked immunosorbent assay (ELISA) confirmed elevated expression of IL-6 in the peritoneal lavage fluid of EPS mice compared to controls (Fig. 2I), highlighting the systemic inflammatory milieu. Another critical aspect of EPS pathology is increased vascular density, often associated with chronic inflammation and fibrosis. In the EPS model, we observed a notable increase in blood vessel density in the visceral peritoneum, as demonstrated by CD31 staining (Fig. 2F, Supplemental Fig. 2C). Collectively, these results provide a comprehensive view of the severe inflammation, fibrosis, and angiogenesis occurring in the peritoneum of EPS mice. Importantly, these pathological changes closely mirror those reported in human EPS, validating the utility of this mouse model for studying disease mechanisms and potential therapeutic interventions.
Dynamic collagen deposition and inflammatory cell infiltration during EPS formation
To investigate the dynamic progression of intraperitoneal adhesion formation in EPS, we sacrificed mice at different time points and analyzed the histopathological changes in the peritoneum. On day 1, no noticeable infiltration of inflammatory cells or collagen fiber deposition was observed on the peritoneal surface. By day 7, inflammatory cells had begun infiltrating the peritoneum, accompanied by the initial deposition of collagen fibers (Fig. 3A). This early infiltration laid the groundwork for adhesion formation, with immune cells accumulating on the surface of fibers by day 14, and the fibrils progressively thickening and interconnecting to form adhesions. As the process advanced, collagen fibers continued to proliferate and gradually became the dominant structural component of the adhesions, while the infiltration of immune cells began to subside. By day 21, the adhesions had evolved into dense fibrous scar tissue, with minimal remaining immune cell infiltration (Fig. 3A). These findings closely mirror the pathological evolution observed in human EPS, where early inflammation gives way to excessive fibrotic deposition over time.
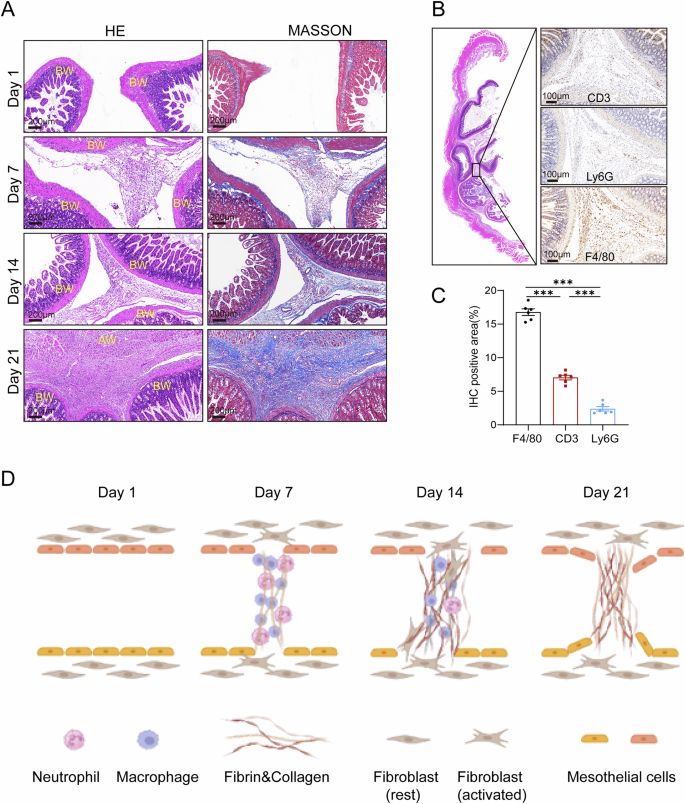
A Representative images of H&E and Masson’s trichrome staining showing the dynamic pathological progression of adhesion formation in EPS mice. Mice were sacrificed at days 1, 7, 14, and 21 to capture different stages of adhesion development (AW: abdominal wall; BW: bowel wall). B Immunohistochemistry staining of adhesion cross-sections showing the distribution of key inflammatory cell types involved in adhesion formation. CD3 was used to identify lymphocytes, Ly6G for neutrophils, and F4/80 for macrophages. C Quantitative analysis of immune cell infiltrations in Fig. 3B (n = 6 per group). D A graphical diagram depicting the dynamic formation of interorgan adhesions, illustrating the transition from early inflammatory cell infiltration to fibrous scar tissue formation over time. Data are presented as mean ± SEM. *** p < 0.001, two-tailed Student’s t-test.
In addition to collagen depositions, H&E and Masson’s trichrome staining revealed significant infiltration of inflammatory cells within the adhesion regions (Fig. 3A). To determine the types of immune cells involved, we conducted immunohistochemical staining with markers for specific cell types at the conclusion of EPS molding (day 21). The markers used were F4/80 for macrophages, CD3 for T lymphocytes, and Ly6G for neutrophils (Fig. 3B). Among these, macrophages were found to be the predominant immune cells present within the adhesions, suggesting their key role in driving the fibrotic response (Fig. 3C). This was further supported by the extensive infiltration of macrophages in the parietal peritoneum (Supplemental Fig. 3), reinforcing the critical involvement of macrophages in the adhesion formation process. The graphical diagram in Fig. 3D illustrates the dynamic formation process of interorgan adhesions, depicting the transition from early inflammatory infiltration to the eventual deposition of fibrous scar tissue. The findings indicate the role of macrophage recruitment in the development of EPS, offering additional understanding of the cellular processes involved in adhesion formation within this disease model.
Transcriptomic alterations in peritoneal tissues of EPS mouse models
To gain insights into the transcriptomic alterations in the peritoneal tissues of EPS mice, we performed bulk RNA sequencing on the visceral peritoneum, using three biological replicates from both the Control and EPS groups (Fig. 4A). Our analysis identified a total of 3,900 upregulated genes and 4,397 downregulated genes in the EPS group compared to controls (adjusted p-value < 0.05). Notably, macrophage-associated genes such as Marco and Csf3r, along with chemokine-related genes, exhibited significant upregulation in the EPS group, underscoring the inflammatory nature of the disease (Supplemental Fig. 4).
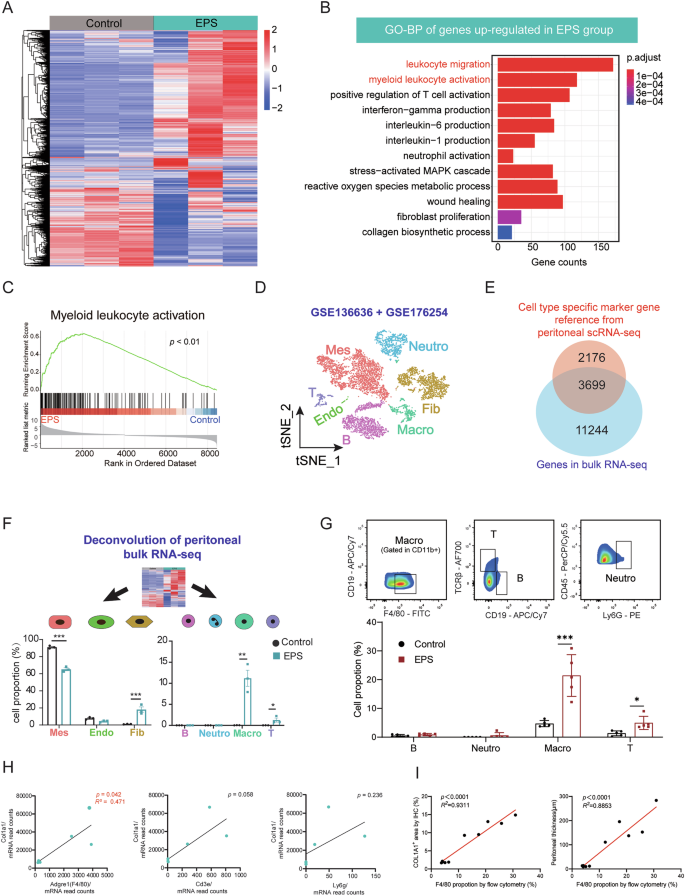
A Heatmap of RNA-seq data comparing gene expression in the visceral peritoneum of Control (n = 3) and EPS (n = 3) mice. B Gene Ontology (GO) biological process enrichment analysis of differentially expressed genes (DEGs) significantly upregulated in EPS mice compared to controls. C GSEA) showing enhanced myeloid leukocyte activation and regulation of leukocyte adhesion in EPS mice. D UMAP plot illustrating cell clusters from two publicly available single-cell RNA sequencing (scRNA-seq) datasets of mouse omentum. Mes: mesothelial cells; Endo: Endothelial cells; Fib: Fibroblasts; Neutro: Neutrophils; Macro: Macrophages; T: T cells. E Venn diagram showing the overlap between cell type-specific marker genes from scRNA-seq datasets and DEGs identified in our bulk RNA-seq data. F Deconvolution analysis using scRNA-seq datasets to compare cellular proportions in the Control and EPS groups, highlighting an increased proportion of fibroblasts among parenchymal cells and macrophages among immune cells in EPS mice (n = 3 per group). G Flow cytometry analysis of visceral peritoneum from intestine, confirming a significant increase in immune cell infiltration, particularly macrophages, in EPS mice compared to controls (n = 5 per group). H Correlation analysis between COL1A1 expression and the infiltration of various inflammatory cell types, showing a strong positive correlation between macrophage infiltration and extracellular matrix (ECM) progression. I Correlation analysis between the macrophages proportion detected by flow cytometry with COL1A1 expression in the peritoneum by immunohistochemistry and peritoneal thickness. Data are presented as mean ± SEM. * p < 0.05, *** p < 0.001, two-tailed Student’s t-test.
To better understand the functional implications of these differentially expressed genes (DEGs), we performed Gene Ontology (GO) enrichment analysis. As shown in Fig. 4B, the upregulated genes in the EPS group were predominantly enriched in biological processes associated with immune activation, such as leukocyte migration, myeloid leukocyte activation, and interleukin production. These findings suggest that immune cell activation, particularly the infiltration of mononuclear macrophages, plays a central role in the progression of EPS. In addition, Gene Set Variation Analysis (GSVA) further supported this, with a marked increase in myeloid leukocyte activation scores in the EPS mice (Fig. 4C). This aligns with the observed increase in macrophage infiltration, indicating that these immune cells are key contributors to the ongoing inflammatory and fibrotic processes.
To gain further insight into the cellular composition of the peritoneal tissues, we utilized two publicly available single-cell RNA sequencing (scRNA-seq) datasets from mouse omentum and classified them into seven distinct clusters based on canonical marker genes (Fig. 4D) (GSE 136636, GSE176254). Differential expression analysis between the Control and EPS groups revealed that 63% of the marker genes identified from scRNA-seq overlapped with those from our bulk RNA-seq data (Fig. 4E). Using this dataset, we applied deconvolution analysis with the R package MuSiC to assess the relative proportions of different cell types. Our analysis showed a marked decrease in the proportion of mesothelial cells in the EPS group, suggesting a disruption in the mesothelial layer, which is a known contributor to adhesion formation by exposing adhesive fibrin clots to surrounding tissues23. Conversely, we observed a significant increase in the proportion of fibroblasts in the EPS mice, which likely contributes to the excessive fibrous deposition seen in the disease. In terms of immune cell populations, both macrophages and T cells were significantly elevated in the EPS group, with macrophages being the most predominant immune cell type (Fig. 4F). To validate these findings, we performed flow cytometry on peritoneal tissues, confirming that the percentage of macrophages was significantly higher in EPS mice, corroborating the RNA-seq results (Fig. 4G). Furthermore, we examined the correlation between different immune cell types and the expression of the fibrosis marker Col1a1. Among the immune cell markers evaluated, F4/80, a macrophage marker, demonstrated the strongest positive correlation with Col1a1 expression (R2 = 0.471, p = 0.042) (Fig. 4H). Concurrently, the proportion of macrophages detected by flow cytometry showed a significant positive correlation with COL1A1 expression in the peritoneum as measured by immunohistochemistry (R2 = 0.9311, p < 0.0001), as presented in Fig. 4I, suggesting that macrophages are the main immune cell type involved in fibrotic deposition in EPS. These findings, when combined with our previous immunohistochemistry data, emphasize the pivotal role of macrophage infiltration in the formation of EPS and further highlight the synergistic relationship between immune cell infiltration and fibrotic deposition in this disease model.
Activation of the cGAS-STING pathway in mesothelial cells regulates macrophage chemotaxis
To investigate the mechanism underlying the significant macrophage infiltration observed in the EPS peritoneum, we reanalyzed our bulk RNA sequencing data. KEGG analysis revealed that genes upregulated in EPS mice were enriched in pathways related to cell chemotaxis and cytoplasmic DNA sensing, particularly the cGAS–STING pathway (Supplemental Fig. 5A–B), highlighting a potential link between immune cell recruitment and intracellular immune surveillance mechanisms (Fig. 5A). Additionally, GSVA demonstrated a strong positive correlation between the cytoplasmic DNA-sensing pathway and cytokine chemotaxis and inflammatory response pathways, further supporting the role of intracellular surveillance system in EPS progression (Fig. 5B, C).
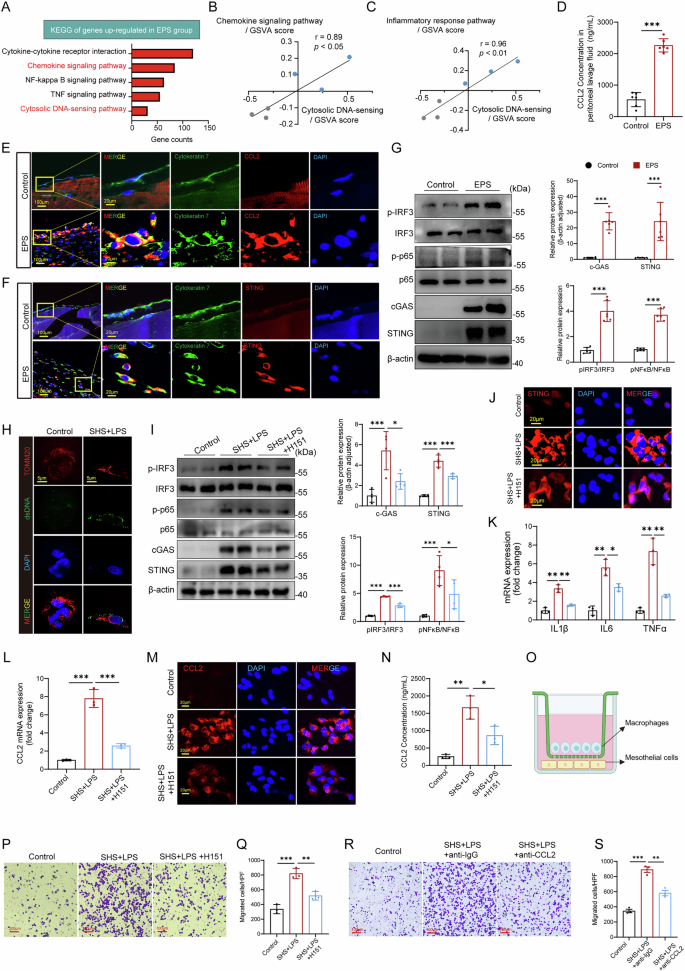
A KEGG analysis showing significant enrichment of upregulated genes in cell chemotaxis and cytoplasmic DNA-sensing pathways in EPS mice. GSVA scores demonstrating a positive correlation between the cytoplasmic DNA-sensing pathway and cytokine chemotaxis (B) and inflammatory response pathways (C). D ELISA results showing elevated CCL2 levels in the peritoneal lavage fluid of EPS mice (n = 6 per group). E Immunofluorescence indicating increased CCL2 expression in the EPS parietal peritoneum, primarily co-localized with Cytokeratin 7+ mesothelial cells. F Immunofluorescence showing increased STING expression in the EPS parietal peritoneum, also co-localized with Cytokeratin 7+ mesothelial cells. G Western blot confirming the activation of cGAS-STING and downstream proteins in the EPS peritoneum (n = 6 per group). H Cytoplasmic DNA leakage observed in mesothelial cells stimulated with LPS + SHS. I, J Western blot and immunofluorescence showing STING activation and downstream signaling in mesothelial cells under EPS stimulation, with H151 reducing this activation (n = 4 independent experiments). K qPCR showing upregulation of inflammatory-related genes in mesothelial cells under EPS stimulation, with or without H151 pretreatment (n = 3 independent experiments). L qPCR demonstrating increased CCL2 gene expression in mesothelial cells under EPS stimulation, with H151 reducing the effect (n = 3 independent experiments). M Immunofluorescent staining of CCL2 in EPS-stimulated mesothelial cells. N ELISA showing elevated CCL2 levels in mesothelial cell supernatant under EPS stimulation, with H151 reducing CCL2 secretion (n = 3 independent experiments). O Schematic of the trans-well assay to assess macrophage migration. Mesothelial cells were seeded in the lower chamber, stimulated with EPS, and co-cultured with macrophages from the upper chamber (Created in BioRender. Sun, J. (2025) https://BioRender.com/vbajaol). P Bright-field image of macrophage migration after co-culturing with EPS-stimulated mesothelial cells. Q Quantification of macrophage migration under different conditions, with H151 alleviating macrophage migration (n = 3 independent experiments). R, S Representative image and quantification of macrophage migration under anti-CCL2 treatment, (n = 3 independent experiments). Data are presented as mean ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001, two-tailed Student’s t-test.
Given that CCL2 is a well-established chemokine involved in macrophage recruitment24,25, our qPCR validation confirmed that CCL2 exhibited the most pronounced expression change among chemokines in the peritoneal tissues of EPS mice (Supplemental Fig. 6A). ELISA analysis of the peritoneal lavage fluid revealed significantly higher levels of CCL2 in the EPS group compared to controls (Fig. 5D). Immunofluorescence also showed a marked increase in CCL2 expression in the parietal peritoneum, primarily co-localizing with Cytokeratin 7+ mesothelial cells (Fig. 5E, Supplemental Fig. 6B). These findings suggest that mesothelial cells are the primary source of CCL2 secretion in EPS, driving the chemotaxis of macrophages.
We then investigated the activation of the cGAS-STING pathway, which is known to mediate responses to cytoplasmic DNA. Both Western blot and immunofluorescence confirmed significant activation of the cGAS-STING pathway in EPS peritoneum, with STING co-localizing primarily with mesothelial cells (Fig. 5F, G, Supplement Supplemental Fig. 6C, D), suggesting that activation of the cGAS-STING pathway in mesothelial cells under EPS conditions may be responsible for promoting the chemotaxis of inflammatory cells, such as macrophages. After initial experiments to set up the in vitro EPS model (Supplemental Fig. 6E-H), we simulated the EPS environment by treating human peritoneal mesothelial cells (HPMCs) with LPS and SHS. Under these conditions, mesothelial cells exhibited cytoplasmic DNA leakage (Fig. 5H), consistent with cGAS-STING pathway activation. Western blotting and immunofluorescence confirmed the activation of STING and its downstream proteins in mesothelial cells upon EPS stimulation, while treatment with the STING inhibitor H151 partially mitigated this effect (Fig. 5I, J). qPCR analysis further demonstrated that the expression of inflammatory-related genes was significantly upregulated in mesothelial cells under EPS stimulation, and this effect was also suppressed by H151 (Fig. 5K). We next examined the role of CCL2 in this process. RT-qPCR and cellular immunofluorescence showed that both CCL2 gene expression and CCL2 protein levels were significantly elevated in mesothelial cells under EPS stimulation, and these increases were attenuated by H151 (Fig. 5L, M). The secretion of CCL2 in the cell supernatant was also significantly higher in EPS-stimulated mesothelial cells (Fig. 5N). STING agonists ADU-S100 also significantly induced CCL2 expression (Supplemental Fig. 6I, J), verifying the role of STING activation in promoting CCL2 secretion.
To directly assess the impact of mesothelial cell activation on macrophage migration, we performed a transwell co-culture assay, where mesothelial cells were co-cultured with macrophages (Fig. 5O). EPS-stimulated mesothelial cells significantly promoted macrophage migration (Fig. 5P, Q), while treatment with H151 partially alleviated this effect. These findings demonstrate that activation of the cGAS-STING pathway in mesothelial cells leads to the secretion of CCL2, which in turn induces macrophage chemotaxis and migration. Collectively, our results highlight the critical role of mesothelial cells in sensing EPS-related stimuli and activating the cGAS-STING pathway, thereby driving macrophage recruitment through CCL2 secretion. Inhibition of STING effectively reduces both CCL2 production and macrophage migration, offering a potential therapeutic approach for mitigating macrophage-driven inflammation in EPS. Additionally, we observed that anti-CCL2 intervention also significantly attenuated in vitro EPS-induced macrophage migration, again indicating that CCL2 acts as the predominant cytokine driving macrophage chemotaxis in this microenvironment (Fig. 5R, S).
Inhibition of cGAS-STING activation ameliorates EPS formation in mice
To investigate whether inhibiting the cGAS-STING pathway could reduce the severity of EPS, we administered the STING inhibitor H151 via intraperitoneal injection in mice (Fig. 6A). Western blot and immunofluorescence analyses confirmed that H151 effectively suppressed the activation of STING and downstream signaling proteins in the peritoneal tissues (Fig. 6B), validating its ability to block STING signaling in vivo. In parallel, H151 treatment significantly lowered the expression of CCL2 in peritoneal tissue (Fig. 6C, Supplemental Fig. 7A) and in the peritoneal lavage fluid (Fig. 6D). This reduction in CCL2 was associated with a decrease in macrophage infiltration on the peritoneal surface, observed through immunohistochemistry (Fig. 6E, F). Correlation analysis between the CCL2 concentration in peritoneal lavage fluid and the F4/80 infiltration area showed a significant positive correlation (Fig. 6G). These results indicate that STING-mediated chemotaxis, driven by CCL2, plays a central role in recruiting macrophages during EPS progression. Therapeutically, H151 treatment markedly improved the pathological features of EPS. Mice treated with H151 showed a significant reduction in adhesion scores during gross assessments (Fig. 6H, I), reflecting decreased intra-abdominal adhesions. Histological staining also revealed a notable reduction in collagen fiber deposition in the adhesion regions (Fig. 6Ja-b and 6K). The thickness of the parietal peritoneum, a hallmark of fibrosis in EPS, was significantly decreased in H151-treated mice (Fig. 6J-c and L), indicating a direct impact on fibrotic progression. Immunohistochemistry showed that ECM-related proteins (COL1A1, fibronectin, α-SMA) (Fig. 6d-f), inflammatory markers (IL-1β, IL-6, TNF-α), and pro-angiogenic VEGF were significantly reduced in the H151-treated group (Fig. 6M), supporting the inhibitor’s anti-adhesion effect. In summary, these findings suggest that inhibiting cGAS-STING activation with H151 reduces CCL2 secretion and macrophage recruitment, ultimately mitigating the excessive collagen deposition and adhesion formation characteristic of EPS. This highlights the potential of targeting the STING pathway as a therapeutic strategy for EPS.
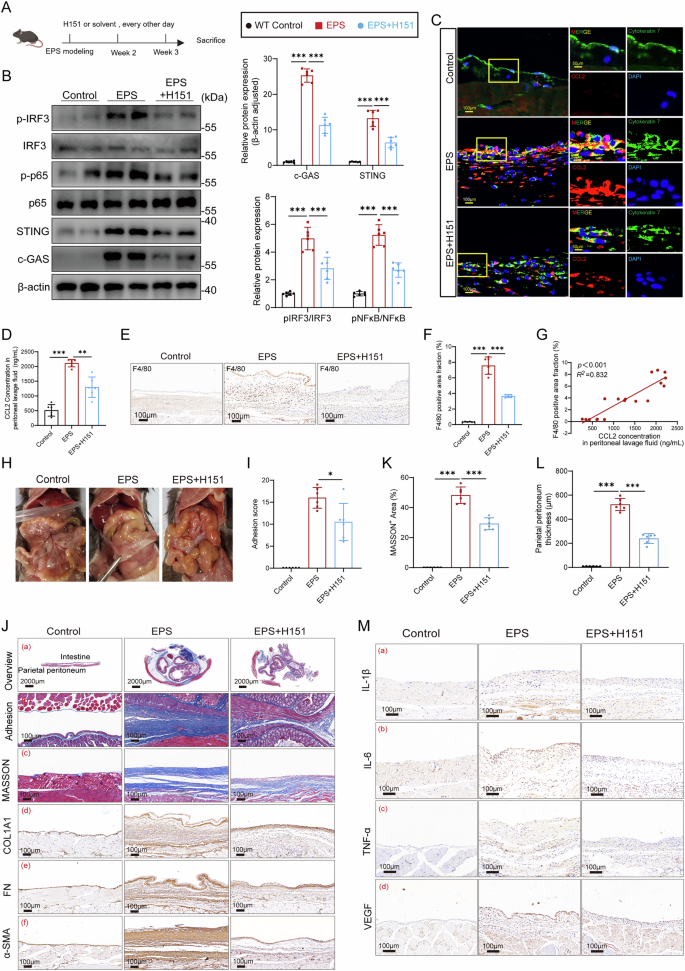
A The schematic diagram of administering the STING inhibitor H151 (Created in BioRender. Sun, J. (2025) https://BioRender.com/vbajaol). Western blot (B) and immunofluorescence (C) demonstrating that H151 effectively reduced the activation of STING and its downstream proteins in peritoneal tissues (n = 6 per group). D ELISA exhibited that H151 partially lowered the CCL2 concentration in peritoneal lavage fluids (n = 6 per group). E–F Immunohistochemistry (IHC) showed H151 effectively reduced macrophage infiltration (n = 6 per group). G Correlation analysis between the CCL2 concentration in peritoneal lavage fluid and the F4/80 infiltration from (F) among the 3 groups. Gross macroscopic viewing of abdomen (H) and the macroscopic adhesion score (I) demonstrated from a macro perspective that H151 effectively alleviated abdominal adhesion (n = 6 per group). J–L Histopathological assessment of intra-abdominal adhesions and fibrous deposition on peritoneal surface. a MASSON staining of the abdominal cross-sections presented the adhesion condition in different groups, and the MASSON+ area (b) was calculated and the statistic diagram is shown in Figure K (n = 6 per group). c Masson’s trichrome staining illustrates the thickness of the parietal peritoneum among the three groups, with (L) showing the corresponding statistical bar graph (n = 6 per group). d–f Immunohistochemical images presenting the expression of three classic ECM-related proteins in parietal peritoneum: COL1A1, Fibronectin, and α-SMA. M Immunohistochemical analysis of inflammatory markers IL-1β, IL-6, and TNF-α in the parietal peritoneum. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, two-tailed Student’s t-test.
IRF3 as the key transcription factor promoting CCL2 secretion in mesothelial cells
Cyclic GMP-AMP synthase (cGAS) detects cytosolic DNA during cellular stress and activates the adaptor protein STING, which triggers immune responses by activating the downstream transcription factor IRF326,27,28. We investigated whether the activation of STING in mesothelial cells under EPS condition regulates CCL2 secretion via IRF3. Using the Univariate Linear Model (ULM) method in the DecoupleR package, we analyzed our bulk RNA-seq data and found that IRF3 transcriptional activity was significantly elevated in the EPS group (Fig. 7A). This increase in IRF3 activity aligns with our observation of increased phospho-IRF3 levels in mesothelial cells under EPS condition (Fig. 5I). Among the genes predicted to be regulated by IRF3, CCL2 and CCL5 were the most upregulated in the EPS group (Fig. 7B), with CCL2 had the higher p-value (p < 10-6). To further investigate how IRF3 promotes CCl2 expression, we used the JASPAR database to analyze the transcription factor binding sites within the CCL2 promoter and identified a high-scoring potential IRF3 binding site (Fig. 7C). This suggested a direct interaction between IRF3 and the CCL2 promoter region (Fig. 7D). To test this possibility, we conducted a chromatin immunoprecipitation (ChIP) assay, which validated that IRF3 indeed binds to the promoter of the CCL2 gene (Fig. 7E, F, Supplemental Fig. 8). These findings demonstrate that under EPS condition, IRF3 binding to the CCL2 promoter facilitates increased CCL2 production and secretion. Thus, IRF3 serves as a key transcription factor driving CCL2 secretion in mesothelial cells in response to EPS. IRF3 knockdown significantly reduced both the transcription and secretion of CCL2 in response to stimulation with a STING agonist (Supplemental Fig. 6I, J) and EPS-conditioned media (Fig. 7G, H). Furthermore, this genetic intervention markedly inhibited macrophage migration, as shown by transwell assays (Fig. 7I, J). These results provide functional evidence that IRF3 is a key transcriptional regulator of CCL2 in EPS-associated mesothelial responses.
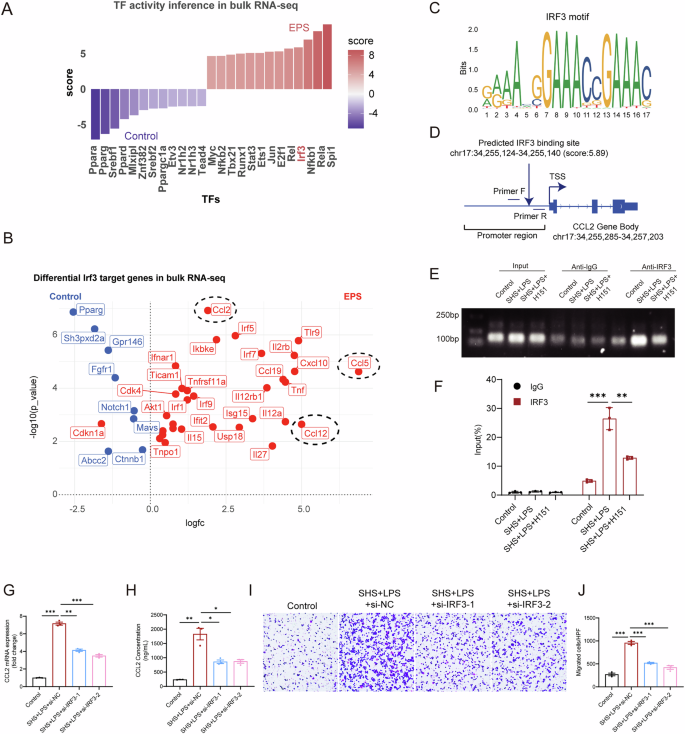
A Bar plot showing the activity scores of differentially expressed transcription factors in Saline and EPS group, based on bulk RNA-seq data. B Volcano plot displaying target genes of IRF3 that are differentially expressed between Saline and EPS group. Red dots indicate genes activated by IRF3, while blue dots represent genes inhibited by IRF3. C Predicted IRF3 binding motifs identified using the JASPAR database. D Schematic illustration showing the IRF3 binding motif within the promoter region of the CCL2 gene. E–F ChIP assays and ChIP-qPCR analysis of IRF3 binding to CCL2 in mesothelial cells treated with or without EPS (SHS + LPS) and H151 (n = 3 independent experiments). Gene silencing of IRF3 significantly reduced both the transcriptional expression (G) and secretion (H) of CCL2 induced by SHS + LPS. I–J Trans-well experiments demonstrating IRF3 silencing suppressed EPS-induced macrophage migration. Data are presented as mean ± SEM. ***p < 0.001, two-tailed Student’s t-test.
Clinical relevance of STING activation and EPS
For a clinical investigation of the cGAS-STING pathway, we collected peritoneal tissues, including adhesion tissues, from patients with EPS and healthy controls. Immunofluorescence staining revealed significant activation of the cGAS-STING pathway in mesothelial cells from the peritoneal surface of EPS patients (Fig. 8A), along with a marked increase in CCL2 expression (Fig. 8B). Given that a significant number of mesothelial cells are shed into the peritoneal dialysis fluid under EPS condition, we also prepared smears of the shed cells from the peritoneal dialysis effluent for immunofluorescence staining, which yielded results consistent with the peritoneal tissue (Fig. 8C–H). Additionally, ELISA measurements confirmed that the concentration of CCL2 in the peritoneal dialysis effluent was significantly higher in EPS patients compared to controls (Fig. 8I), so as the cGAMP, which serves as an indicator of STING activation (Fig. 8J). Furthermore, there is a positive correlation between CCL2 and cGAMP concentrations (Fig. 8K). These clinical findings corroborate the link between STING activation and increased CCL2 levels in EPS, further supporting the role of the cGAS-STING pathway in driving macrophage chemotaxis and inflammation.
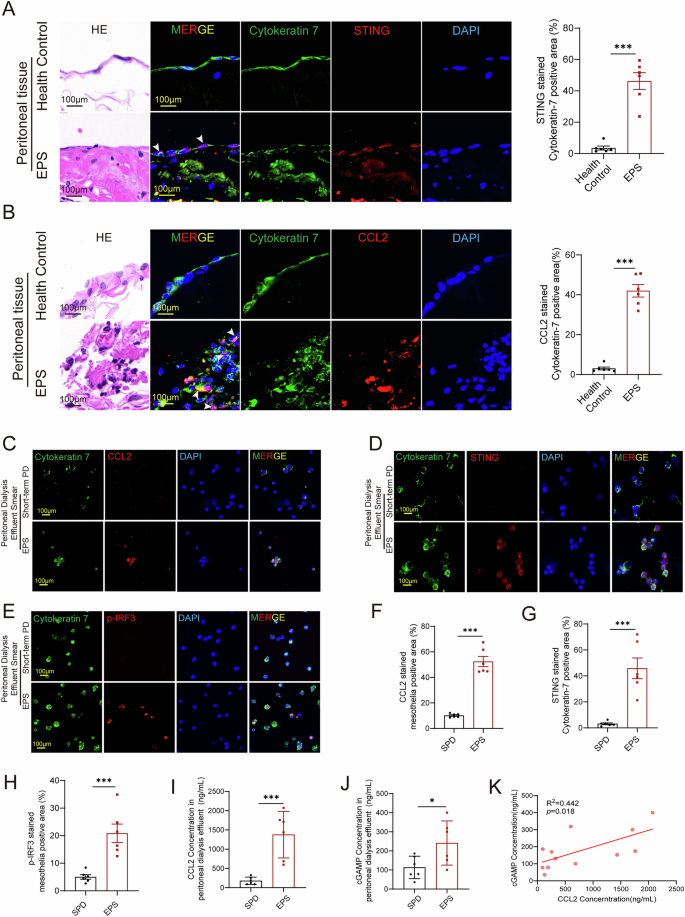
HE staining and immunofluorescence of peritoneal tissue from clinical patients showed the increased expression of STING (A) and CCL2 (B) in EPS patients compared to healthy control (n = 6 per group). C–H Immunofluorescence of peritoneal dialysis effluent smear showed the increased expression of CCL2 (C), STING (D), and p-IRF3 (E) in EPS patients compared to short-term PD patients (SPD) (n = 6 per group). With the corresponding quantitative statistics of fluorescence co-localization showing in F, G and H. I–J ELISA measurements revealed a significant increased CCL2 concentrations (E) and cGAMP concentrations (F) in the peritoneal dialysis effluent in EPS patients compared to control (n = 6 per group). K Correlation analysis presented a significant positive correlation between cGAMP and CCL2 (n = 12). Data are presented as mean ± SEM. *p < 0.05, ***p < 0.001, two-tailed Student’s t-test.