Image Credit: © Tatsiana – stock.adobe.com
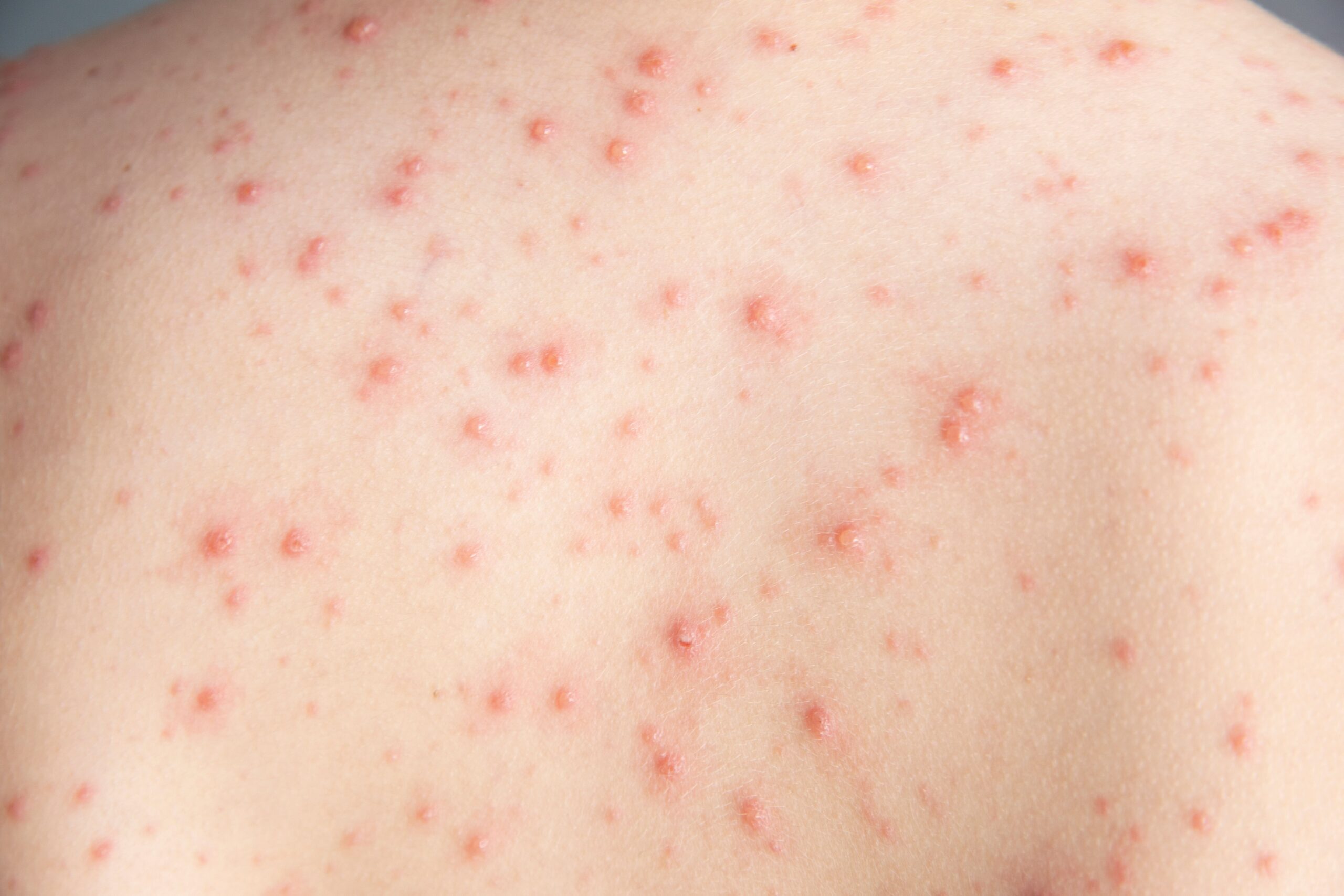
In a move that could revolutionize how acne is treated, Sanofi has launched early-stage clinical trials for an investigational mRNA acne vaccine, a first-of-its-kind approach that leverages the same technology used in COVID-19 vaccines. With over 50 million Americans affected by acne each year and current treatments often falling short, this novel vaccine has generated considerable anticipation among dermatologists and patients alike.
Sanofi began its phase 1 and 2 trials in 2024, aiming to enroll approximately 400 healthy adults between the ages of 18 and 45 who suffer from moderate to severe facial acne. The study (NCT06316297) is expected to continue through 2027. Participants will receive up to 3 doses of the mRNA-based vaccine or a placebo to evaluate not only how well the vaccine reduces acne symptoms but also to monitor safety and determine the ideal dosing for future studies.
To understand the significance of this development, Dermatology Times spoke with Joshua Zeichner, MD, Associate Professor of Dermatology and Director of Cosmetic & Clinical Research in Dermatology at Mount Sinai Hospital in New York City.
“mRNA is like an instruction manual that tells our cells to produce specific proteins. In the form of a vaccine, mRNA strands are taken up by our cells and tell our cells to produce specific proteins that then elicit an immune response and protect our body against the external factor associated with that protein,” Zeichner explained.

While most vaccines aim to protect against potentially life-threatening infections, the concept of using one to target a common dermatological condition like acne may seem unconventional. But as Zeichner points out, acne, including associated sequelae and scarring, is far more than just a cosmetic nuisance.
“Acne is the most common skin condition we see as dermatologists, affecting up to 85% of teenagers and many adults, and the effects of acne are much more than skin deep, because it can have a long-lasting psychosocial impact,” he said. “We know that acne can impair quality of life, interfering with performance in school and in the workplace. It’s associated with feelings of insecurity and depression and can have a negative impact on interpersonal relationships, so a safe and effective treatment is really important.”
Despite a wide range of available treatments, from topical retinoids and benzoyl peroxide to oral antibiotics and hormonal therapies, there is no universal cure. A vaccine would offer a fundamentally new approach, not just treating acne after it appears, but preventing the inflammatory process that causes it. So how exactly does an mRNA acne vaccine work?
“For other common dermatological conditions like atopic dermatitis and psoriasis, the main systemic treatments address the abnormal inflammation that leads to these diseases by modulating immune response,” Zeichner explained “What differs about acne is that the driver of inflammation is the Cutibacterium acnes so we need to modulate the inflammatory response against the inflammation-driving strains of the bacteria without disrupting the body’s microbiome.
The vaccine doesn’t aim to eliminate C. acnes entirely, which could disrupt the skin’s microbiome. Instead, it trains the immune system to respond differently to the pro-inflammatory strains, essentially preventing the body from overreacting in the first place.
“So we can think of the vaccine, not so much as a fire extinguisher, putting out the inflammation itself, but rather cutting the fuel source to begin with, and preventing our bodies from mounting that harmful inflammatory response,” he added.
As promising as the science is, Zeichner cautions that the acne vaccine will likely face public scrutiny. Vaccination remains a sensitive topic for many, particularly when it comes to non-fatal conditions.
“I think it’ll take some time for the public to get used to the idea of a vaccine for a common condition like acne versus a potentially life-threatening infection. But this acne treatment is the first of its kind that addresses our body’s immune response,” he commented.
Early results are not yet available, though some encouraging data have emerged from mouse models. Until more is known, clinicians like Zeichner remain hopeful but reserved.
“I’m cautiously optimistic,” Zeichner said. “It’s similar to how people initially viewed electric cars. That was a new form of transportation that took some time getting used to. The acne vaccine seems foreign right now, but after the safety and efficacy are proven, it may become commonplace.”
If successful, Sanofi’s vaccine could usher in a new era in dermatology, not only for acne but potentially for other inflammatory skin conditions where immune system modulation plays a role. While the timeline to market availability remains several years away, the progress marks a significant step forward in how we think about treatment for one of the world’s most common skin diseases.