The addition of daratumumab (Darzalex) to carfilzomib (Kyprolis) plus lenalidomide (Revlimid), and dexamethasone (KRd) should be considered a new standard of care for the treatment of patients with newly diagnosed multiple myeloma who are receiving initial KRd backbone therapy that is independent of transplant eligibility, according to C. Ola Landgren, MD, PhD.1
Data from the phase 2 ADVANCE trial (NCT04268498)
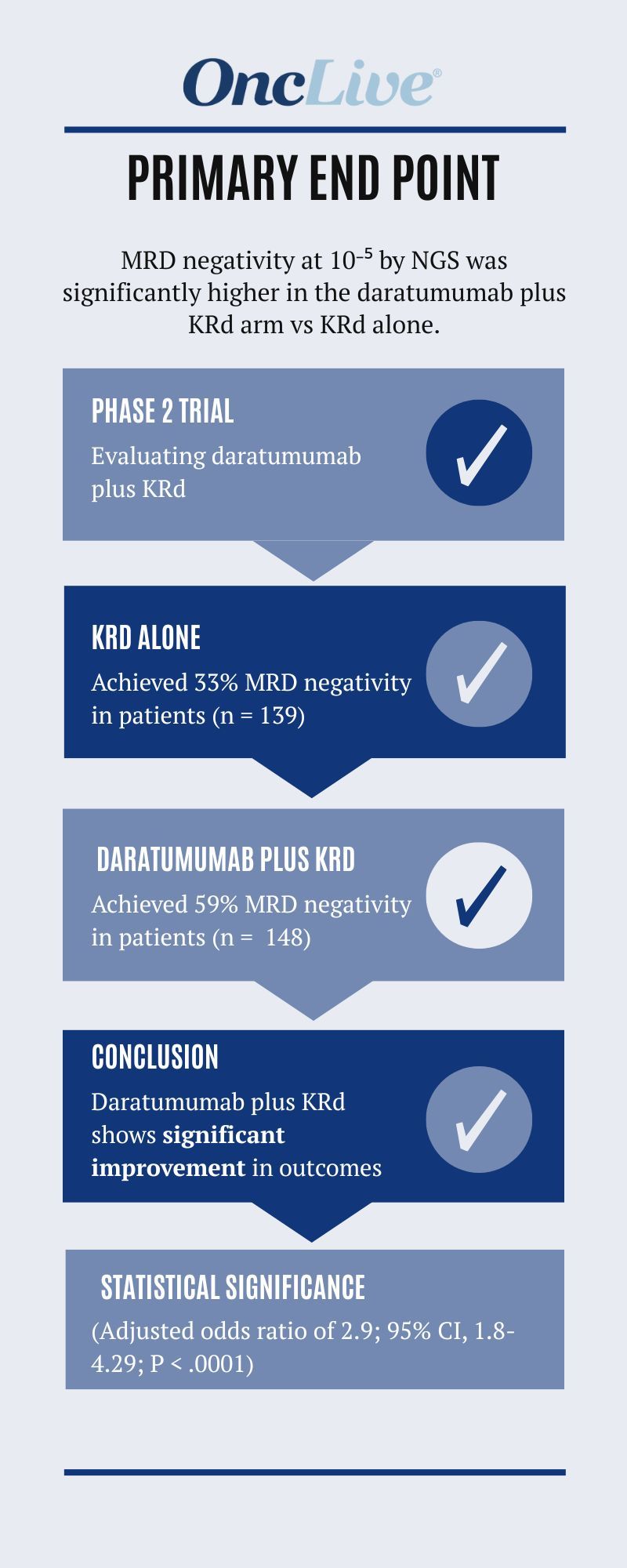
Of note, data from the phase 2 ADVANCE trial (NCT04268498) revealed that the primary end point of minimal residual disease (MRD) negativity rate at a threshold of 10–5 per next-generation sequencing (NGS) in the intention-to-treat population was significantly greater in the daratumumab plus KRd arm (n = 148) at 59% compared with 33% in the KRd arm (n = 139; adjusted odds ratio, 2.9; 95% CI, 1.8-4.29; P < .0001).
“Also, importantly, we used anticoagulation with oral factor Xa inhibition, and we also used minimal intravenous [IV] fluid. We had only 250 mL of saline before therapy for the first cycle to minimize the risk for congestive heart failure and other issues,” Landgren said during an interview with OncLive®. “We saw minimal toxicity with this regimen; it was well tolerated.”
In the interview, Landgren discussed the rationale for the initiation of ADVANCE, the methods of the study, including its unique primary end point, and key efficacy and safety data.
Landgren is the chief of the Division of Myeloma, Department of Medicine, the director of the Sylvester Myeloma Institute, coleader of the Translational and Clinical Oncology Program, the Paul J. DiMare Endowed Chair in Immunotherapy, and a professor at the University of Miami Miller School of Medicine in Florida.
OncLive: What was the rationale of the ADVANCE trial?
Landgren: The multicenter, randomized ADVANCE clinical trial [enrolled] newly diagnosed patients with multiple myeloma [and] compared KRd [with or without] daratumumab. The background for this study was based on modern combination therapies in newly diagnosed multiple myeloma continuing to deliver deep and durable responses reflected in high rates of MRD negativity, and this is found independent of transplant eligibility for patients in the newly diagnosed setting.
Also, with the development of MRD negativity, which we know is associated with progression-free survival [PFS] and overall survival [OS] as an early end point for accelerated approval, which was accomplished [in April 2024] at the [FDA’s] Oncologic Drugs Advisory Committee [ODAC] [meeting]2, there’s an opportunity to design clinical trials for newly diagnosed patients with myeloma, independent of transplant eligibility. In this context, the current ADVANCE trial was designed to offer [patients with] newly diagnosed multiple myeloma access to modern and effective combination therapy independent of transplant eligibility.
What were the key methods of the study and notable baseline patient characteristics of ADVANCE?
ADVANCE was open for all patients with newly diagnosed multiple myeloma, [who are] transplant eligible, transplant ineligible, and transplant deferred. This was possible to do because the primary end point was set as MRD negativity rate at [a threshold of] 10–5 after completion of 8 cycles [of therapy]. Therefore, you could include any patient category, because you could test the end point that way.
The study included 8 cycles of combination therapy, and these were 28-day cycles, comparing daratumumab [plus] KRd as the experimental arm to the backbone of KRd. Some of the highlights are that daratumumab was given subcutaneously per the FDA label. Carfilzomib was given at a dose of 56 mg/m2 via a weekly dosing schedule, and lenalidomide was given at standard 25-mg dosing [from] day 1 [through 14 in a] 21-day cycle. The dexamethasone doses were 20 mg or 40 mg, depending on patients being above or below the threshold of 65 [years of age] or for tolerability [for] dose reductions.
Patients were encouraged to [have their stem cells collected] after 4 cycles, if they were transplant eligible. The [primary] end point was [assessed] after 8 cycles [of treatment]. Therefore, patients had the option, if they were MRD positive after cycle 8, to go to transplant. However, for patients who were MRD negative, the transplant was, by default, deferred per the study protocol, and all the patients moved on to maintenance thereafter, and that was planned for 2 years, or lenalidomide on the protocol, with the option to continue after protocol, standard of care.
What were the most notable efficacy data?
For patients treated with the daratumumab plus KRd regimen, 59% of patients achieved MRD negativity. For patients treated with a KRd regimen, 33% of the patients achieved MRD negativity. The odds ratio was 2.5, and the P value was less than .0001. This is highly significant in favor of the use of the 4 drugs [combined]. [Regarding the] secondary end points, we showed how the MRD negativity at [a threshold] of 10–5 with the NGS assay also showed similar results when we stratified by transplant eligibility and age. We further provided subanalysis [data] where we stratified by high-risk vs standard-risk disease. We also looked at select subtypes of patients, and showed particular benefits in some of the so-called high-risk disease groups. We also provided information on PFS data, but the median follow-up at this time was less than 3 years. We will have to come back and show further follow-up data to show the long-term benefits. However, I would like to emphasize in this context that the work we presented [in April 2024 at the ODAC meeting] for the FDA shows that MRD negativity at [a threshold of] 10–5 is a very strong predictor of long[-term] PFS and OS. Given how strong the MRD results were, we certainly anticipate seeing very strong clinical benefit as well from this study.
The prespecified main result and the subanalysis data for the secondary end points were strikingly superior for the experimental arm [compared with] the control arm. However, another result that is also coming out of this study, when it comes to enrollment. This investigator-initiated randomized multicenter study for [patients with] newly diagnosed multiple myeloma involved 308 patients, and this was done with only 7 independent academic institutions in the US. It also serves as proof of principle that it is possible to do a randomized study in the US in the newly diagnosed setting with very few dedicated institutions. It’s a very important message from the advanced study as well.
What were the key safety characteristics of the ADVANCE regimen?
It’s fair to conclude that these regimens were [both] very safe. Both the daratumumab plus KRd and the KRd regimens showed data very consistent with established safety profiles for each of the individual drugs. It’s also important to emphasize that with the prior discussions on cardiac toxicity and infection-related toxicities with these drugs in prior studies, we didn’t see a lot of cardiovascular problems in this study, and the reason is probably because the study design was optimized. Every patient underwent an echocardiogram and an electrocardiogram, and patients who had underlying cardiovascular disease or were [considered] frail did not participate in this study.
Also, very importantly, in a clinical management note, we gave minimal IV fluid. We gave 250 mL of saline before the dose of the first cycle only, and then we omitted all the IV fluid—that minimized the risk for congestive heart failure. We also gave an oral factor Xa inhibitor. Typically, we gave 10 mg of rivaroxaban [Xalreto] as the anticoagulation medication, and that minimized the risk for any hypocoagulation with deep vein thromboses and pulmonary embolisms. These small measures made such a huge impact, and that’s very important to know that.
References
- Landgren CO, Ye JC, Hillengass J, et al. Randomized, multi-center study of carfilzomib, lenalidomide, and dexamethasone (KRd) with or without daratumumab (D) in patients with newly diagnosed multiple myeloma (NDMM): the ADVANCE clinical trial. J Clin Oncol. 2025;43(suppl 16):7503. doi:10.1200/JCO.2025.43.16_suppl.750
- Oncologic Drugs Advisory Committee (ODAC) Meeting. Combined FDA and Applicants ODAC Briefing Document. Accessed April 12, 2024. https://www.fda.gov/media/177652/download
