Valavanidis, A. & Vlachogianni, T. Plant polyphenols: recent advances in epidemiological research and other studies on cancer prevention. In Studies in Natural Products Chemistry, 39, 269–295. (2013). https://doi.org/10.1016/B978-0-444-62615-8.00008-4
Ahmad, A. et al. Synthesis, photophysical properties and DFT studies of Chalcones and their 2-methoxy-3-cyanopyridine derivatives. J. Photochem. Photobiol Chem. 437, 114494. https://doi.org/10.1016/j.jphotochem.2022.114494 (2023).
Google Scholar
Mahesha, P., Shetty, N. S., Kulkarni, S. D. & Sinha, R. K. A selective bis-thiophene chalcone-based spectrofluorimetric sensor for Fe+3. Luminescence 39, e4823. https://doi.org/10.1002/bio.4823 (2024).
Google Scholar
Hegde, H., Sinha, R. K., Kulkarni, S. D. & Shetty, N. S. Synthesis, photophysical and DFT studies of Naphthyl chalcone and nicotinonitrile derivatives. J. Photochem. Photobiol Chem. 389, 112222. https://doi.org/10.1016/j.jphotochem.2019.112222 (2020).
Google Scholar
Yadav, M., Lal, K., Jose, D. A., Ghule, V. D. & Tittal, R. K. Synthesis, photophysical and DFT investigations on 1,2,3-triazoles linked to chalcone and chalco-pyrene. Chem. Pap. 77, 4457–4467. https://doi.org/10.1007/s11696-023-02794-4 (2023).
Google Scholar
Kumar, C. H. P., Manjunatha, S. K. & Nandeshwarappa, B. P. Synthesis of novel pyrazolic analogues of Chalcones as potential antibacterial and antifungal agents. Curr. Chem. Lett. 12, 613–622. https://doi.org/10.5267/j.ccl.2023.2.001 (2023).
Google Scholar
Lahtchev, K. L., Batovska, D. I., Parushev, S. P., Ubiyvovk, V. M. & Sibirny, A. A. Antifungal activity of chalcones: a mechanistic study using various yeast strains. Eur. J. Med. Chem. 43, 2220–2228. https://doi.org/10.1016/j.ejmech.2007.12.027 (2008).
Google Scholar
Bhade, M. W. Antifungal assay of some novel chalcone derivatives. Curr. Agric. Res. J. 11, 258–264. https://doi.org/10.12944/carj.11.1.22 (2023).
Google Scholar
Bentrad, N. & Hamida-Ferhat, A. Analytical approaches used in profiling natural products with a therapeutic target: a global perspective on nutrition and health. Stud. Nat. Prod. Chem. 72, 57–101. https://doi.org/10.1016/B978-0-12-823944-5.00017-X (2022).
Google Scholar
Mahapatra, D. K., Bharti, S. K., Asati, V. & Singh, S. K. Perspectives of medicinally privileged chalcone-based metal coordination compounds for biomedical applications. Eur. J. Med. Chem. 174, 142–158. https://doi.org/10.1016/j.ejmech.2019.04.032 (2019).
Google Scholar
Raut, N. A., Dhore, P. W., Saoji, S. D. & Kokare, D. M. Selected bioactive natural products for diabetes mellitus. Stud. Nat. Prod. Chem. 48, 287–322. https://doi.org/10.1016/B978-0-444-63602-7.00009-6 (2016).
Google Scholar
Maydt, D., De Spirt, S., Muschelknautz, C., Stahl, W. & Müller, T. J. Chemical reactivity and biological activity of Chalcones and other α, β-unsaturated carbonyl compounds. Xenobiotica 43, 711–718. https://doi.org/10.3109/00498254.2012.754112 (2013).
Google Scholar
Abbo, H. S., Lai, H., Titinchi, S. J. J. & C., & Substituent and solvent effects on UV-visible absorption spectra of chalcone derivatives: experimental and computational studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 303, 123180. https://doi.org/10.1016/j.saa.2023.123180 (2023).
Google Scholar
Gomes, M. N. et al. Chalcone derivatives: promising starting points for drug design. Molecules 22, 1210. https://doi.org/10.3390/molecules22081210 (2017).
Google Scholar
Lemes, S. R. et al. Optical properties and antiangiogenic activity of a chalcone derivative. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 204, 685–695. https://doi.org/10.1016/j.saa.2018.06.099 (2018).
Google Scholar
Winter, C. et al. Activated carbons for chalcone production: Claisen-Schmidt condensation reaction. Chem. Eng. J. 303, 604–610. https://doi.org/10.1016/j.cej.2016.06.058 (2016).
Google Scholar
Sekar, P., Kumar, S. & Raju, S. K. Chemistry and synthetic methodologies of Chalcones and their derivatives: a review. Int. J. Biol. Pharm. Sci. Archive. 5, 51–72. https://doi.org/10.53771/ijbpsa.2023.5.1.0020 (2023).
Google Scholar
Donaire-Arias, A. et al. Synthesis of chalcones: an improved high-yield and substituent-independent protocol for an old structure. Molecules 28, 7576. https://doi.org/10.3390/molecules28227576 (2023).
Google Scholar
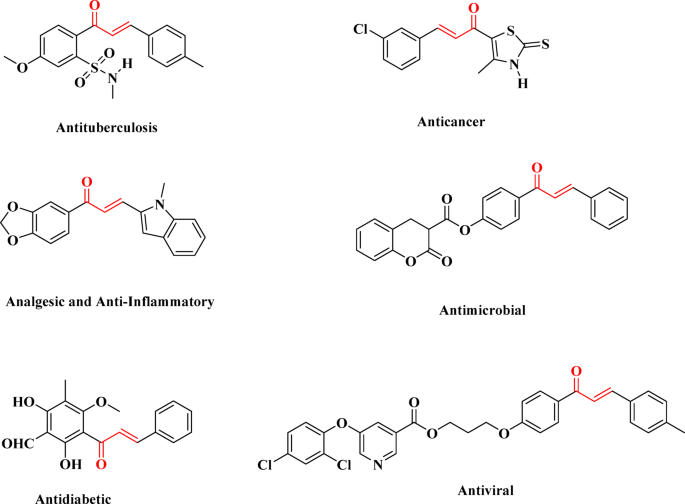
Elkanzi, N. A. A. et al. Synthesis of chalcone derivatives and their biological activities: a review. ACS Omega. 7, 27769–27786. https://doi.org/10.1021/acsomega.2c01779 (2022).
Google Scholar
Mann, G. et al. Bio-evaluation of 99mTc-labeled homodimeric chalcone derivative as amyloid-β-targeting probe. Front. Med. 9, 813465. https://doi.org/10.3389/fmed.2022.813465 (2022).
Google Scholar
Cui, M., Ono, M., Kimura, H., Liu, B. L. & Saji, H. Synthesis and biological evaluation of indole-chalcone derivatives as β-amyloid imaging probe. Bioorg. Med. Chem. Lett. 21, 980–982. https://doi.org/10.1016/j.bmcl.2010.12.045 (2011).
Google Scholar
Mendanha, D. et al. Neves, N. M. A new chalcone derivative with promising antiproliferative and anti-invasion activities in glioblastoma cells. Molecules 26, 3383. https://doi.org/10.3390/molecules26113383 (2021).
Google Scholar
Yang, G., Li, Y., Wang, B. & Zhang, Y. Lighting up fluorescence: precise recognition of halogenated solvents through effective fluorescence detection using chalcone derivatives as a D–A–D–A-type fluorescent chemosensor. J. Fluoresc. 35, 357–368. https://doi.org/10.1007/s10895-023-03527-2 (2023).
Google Scholar
Kamakshi, R. & Reddy, B. S. R. Synthesis of chalcone-based fluorescent polymers: Diels–Alder reaction of Chalcones and their polymerization through ROMP. J. Polym. Sci., Part A: Polym. Chem. 46, 1521–1531. https://doi.org/10.1002/pola.22493 (2008).
Google Scholar
Shkir, M., Patil, P. S., Arora, M., AlFaify, S. & Algarni, H. An experimental and theoretical study on a novel donor–π–acceptor Bridge type 2,4,5-trimethoxy-4′-chlorochalcone for optoelectronic applications: a dual approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 173, 445–456. https://doi.org/10.1016/j.saa.2016.09.022 (2017).
Google Scholar
Ozkan, D. et al. DNA and PNA sensing on mercury and carbon electrodes by using methylene blue as an electrochemical label. Bioelectrochemistry 58, 119–126. https://doi.org/10.1016/S1567-5394(02)00131-7 (2002).
Google Scholar
Wangngae, S. et al. Photophysical study and biological applications of synthetic chalcone-based fluorescent dyes. Molecules 26, 2979. https://doi.org/10.3390/molecules26102979 (2021).
Google Scholar
Prabu, S., Nagalakshmi, R. & Srinivasan, P. Investigations on the physicochemical properties of 4-bromochalcone single crystals for nonlinear optical applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 103, 45–52. https://doi.org/10.1016/j.saa.2012.10.073 (2013).
Google Scholar
Kumari, R. & Milton, M. D. Non-doped, solid-state red-emitting phenothiazine–pyrene chalcones: synthesis, aggregation-induced emission enhancement and mechanofluorochromism. J. Photochem. Photobiol Chem. 462, 116222. https://doi.org/10.1016/j.jphotochem.2024.116222 (2025).
Google Scholar
Chaithanya, B., Chary, D. P. & Anna, V. R. Synthesis and biological evaluation of chalconeincorporated thiazoleisoxazole derivatives as anticancer agents. Chem. Data Collections. 55, 101177. https://doi.org/10.1016/j.cdc.2024.101177 (2025).
Google Scholar
Zheng, M. et al. Synthesis, biological evaluation, and mechanism study of a novel indolepyridine chalcone derivative as an antiproliferative agent against tumor cells through dual targeting tubulin and HK2. Eur. J. Med. Chem. 282, 117058. https://doi.org/10.1016/j.ejmech.2024.117058 (2025).
Google Scholar
Jadhav, S. R., Gurav, S. S., Yasin, H., Nagpal, P. & Mali, S. N. Imidazo[1,2a] pyridineappended chalcone and schiff base conjugates: synthetic, spectrophotometric, biological, and computational aspects. Chem. Phys. Impact. 9, 100694. https://doi.org/10.1016/j.chphi.2024.100694 (2024).
Google Scholar
Saha, A., Karar, M. & Choudhury, S. Red edge effect of chalcone derivatives and their application in biosensing. RSC Adv. 15, 13505–13512. https://doi.org/10.1039/d4ra06978a (2025).
Google Scholar
Frisch, M. J. et al. J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2013.