heic2512 — Science Release
18 December 2025
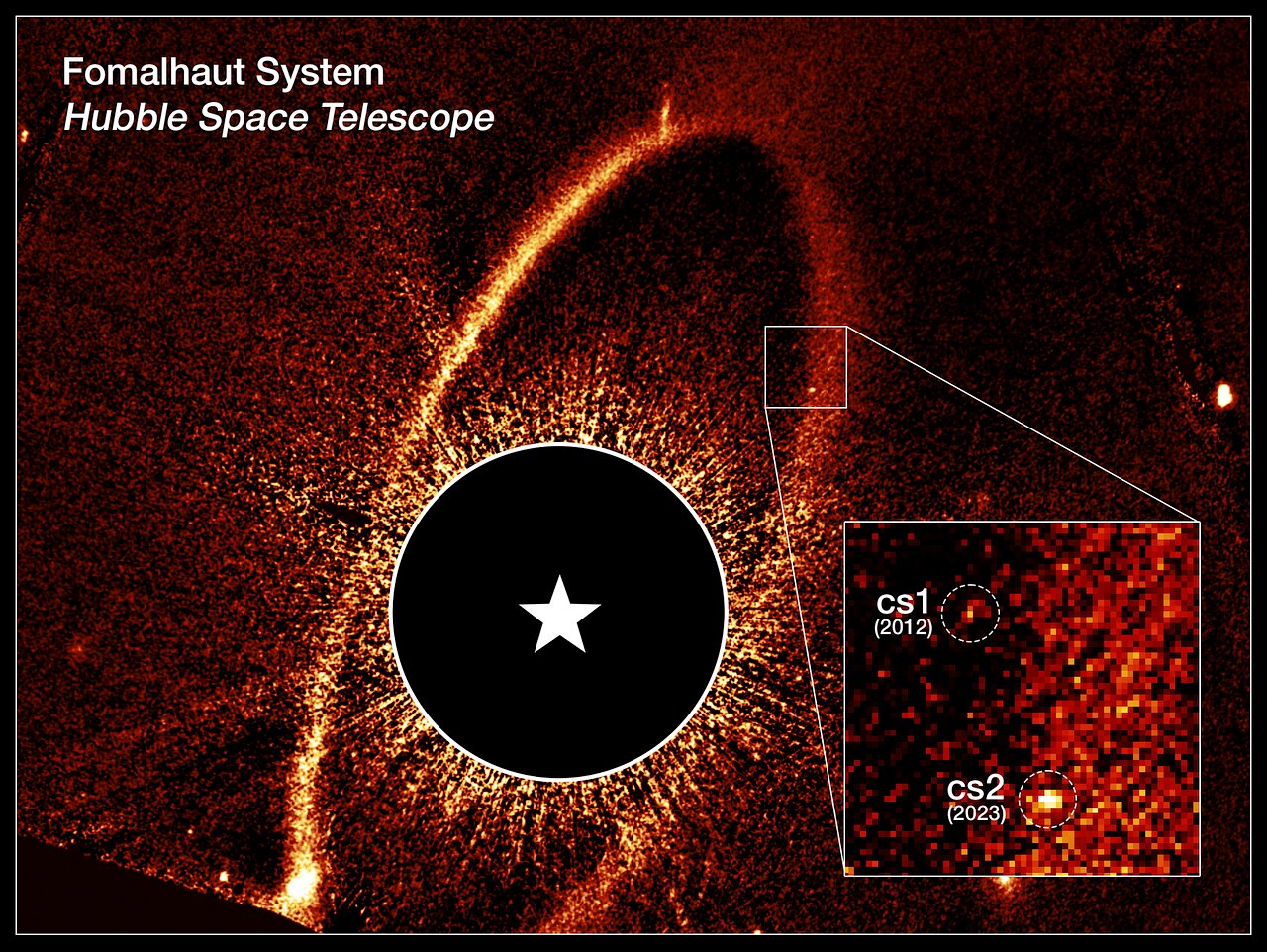
In a historical milestone, catastrophic collisions in a nearby planetary system were witnessed for the first time by astronomers using the NASA/ESA Hubble Space Telescope. As…
18 December 2025
In a historical milestone, catastrophic collisions in a nearby planetary system were witnessed for the first time by astronomers using the NASA/ESA Hubble Space Telescope. As…