Your skin endures constant scratches, scrapes, and bumps, yet the bacterium Staphylococcus aureus can cling on through it all. Although usually harmless, S. aureus can cause serious infections and exacerbate atopic dermatitis. But staph’s stubborn stick wasn’t well understood—until now. An international team of scientists has uncovered interactions between the two proteins that make up the strong bond. The researchers say their findings explain why damaged skin might have a harder time getting rid of the bacteria and could suggest new treatment options (Sci. Adv. 2025, DOI: 10.1126/sciadv.adu7457).
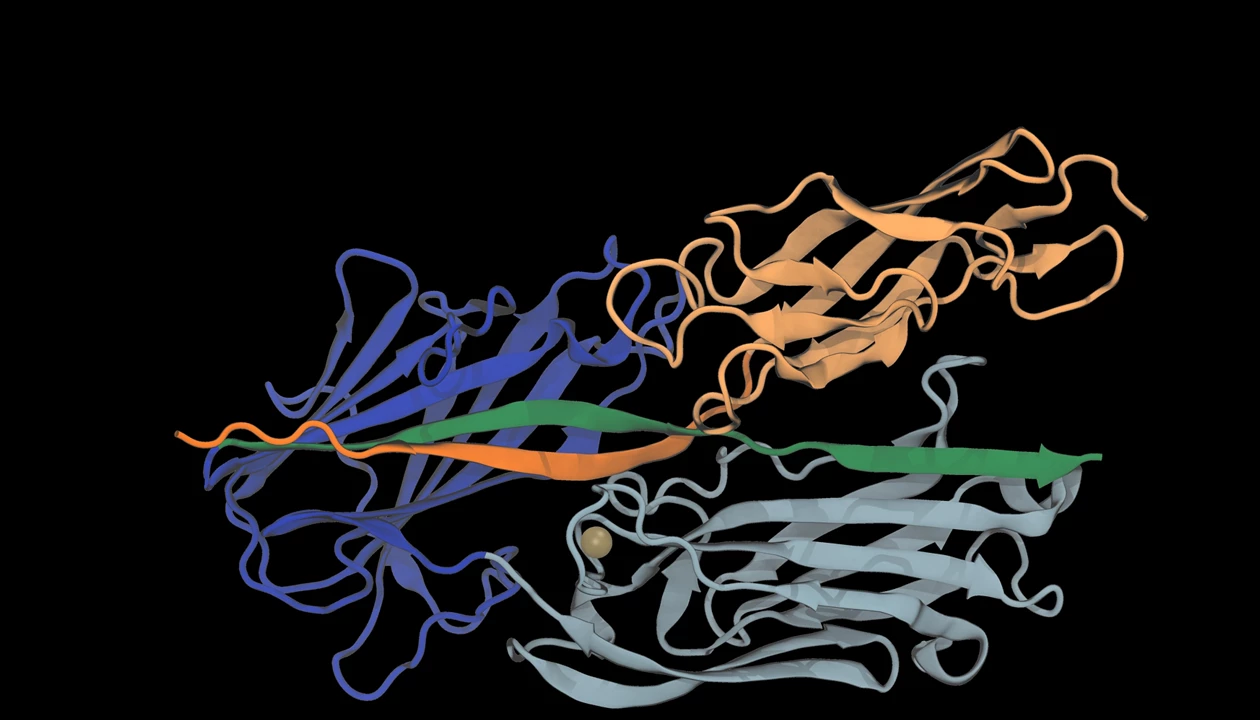
Scientists already knew that S. aureus uses its SdrD protein to bind to a protein that helps skin cells stick together, called desmoglein-1, but the interaction wasn’t fully mapped out. In the new work, “we modeled the whole system,” says Priscila Gomes, a postdoc at Auburn University who was one of the scientists involved. “Now we have the whole picture of this interaction.”
Using atomic force microscopy (AFM), which measures how much force it takes to bend a tiny needle, the team showed that it takes 2 nanonewtons of force to pull away an AFM needle coated with desmoglein-1 from bacteria expressing SdrD. That makes it the strongest noncovalent molecular interaction ever recorded, says Rafael C. Bernardi, a physics professor at Auburn University and leader of the lab that Gomes works in. Most protein bonds will snap with a few piconewtons, he says.
Through computational modeling, the team found that the A domain on SdrD binds to desmoglein-1 through a series of hydrogen bonds and aromatic ring interactions, which together add up to create such a strong hold. Meanwhile, the folded repeats of the protein’s B domains act as mechanical shock absorbers, unfolding when force is applied.
The final component securing SdrD to desmoglein-1 is calcium ions, which stabilize the domains and act as a blockade when the protein is being pulled. The full mechanism means that the more you pull, the more it resists.
“It’s like those toys we had at the state fair where you put your fingers in, and the harder you pull, the tighter it grips,” says Andrew Herr, who studies cellular adhesion and biofilms at the Cincinnati Children’s Hospital Medical Center and who was not involved in the study. The interaction differs from other known bacterial adhesion mechanisms, he says.
Calcium is also a natural part of the wound-healing process and is produced when damage occurs. Knowing the bacteria also uses it to stick may help explain why in experiments with cells affected with atopic dermatitis, SdrD has more adhesion events compared with healthy cells. This is a “really big step forward” in our understanding of the relationship between atopic dermatitis and S. aureus, Herr says.
Bernardi says that knowing exactly how S. aureus achieves its strong stick opens the door to new treatments. For example, instead of killing the bacteria, which can fuel antibiotic resistance, targeting the exact spot they attach could prevent colonization, he says. He also suggests that the findings could provide a strong handle for single-molecule attachments, which could be used for materials or hydrogels.
“This study enhances knowledge by showing in great molecular detail the binding of these two proteins,” says Tim Foster, a retired professor of microbiology at Trinity College Dublin and expert in S. aureus adherence, who was not involved in the study. But because the bacteria use multiple binding mechanisms, whether targeting this interaction will be an effective therapy is tough to say, he says.
Chemical & Engineering News
ISSN 0009-2347
Copyright ©
2025 American Chemical Society