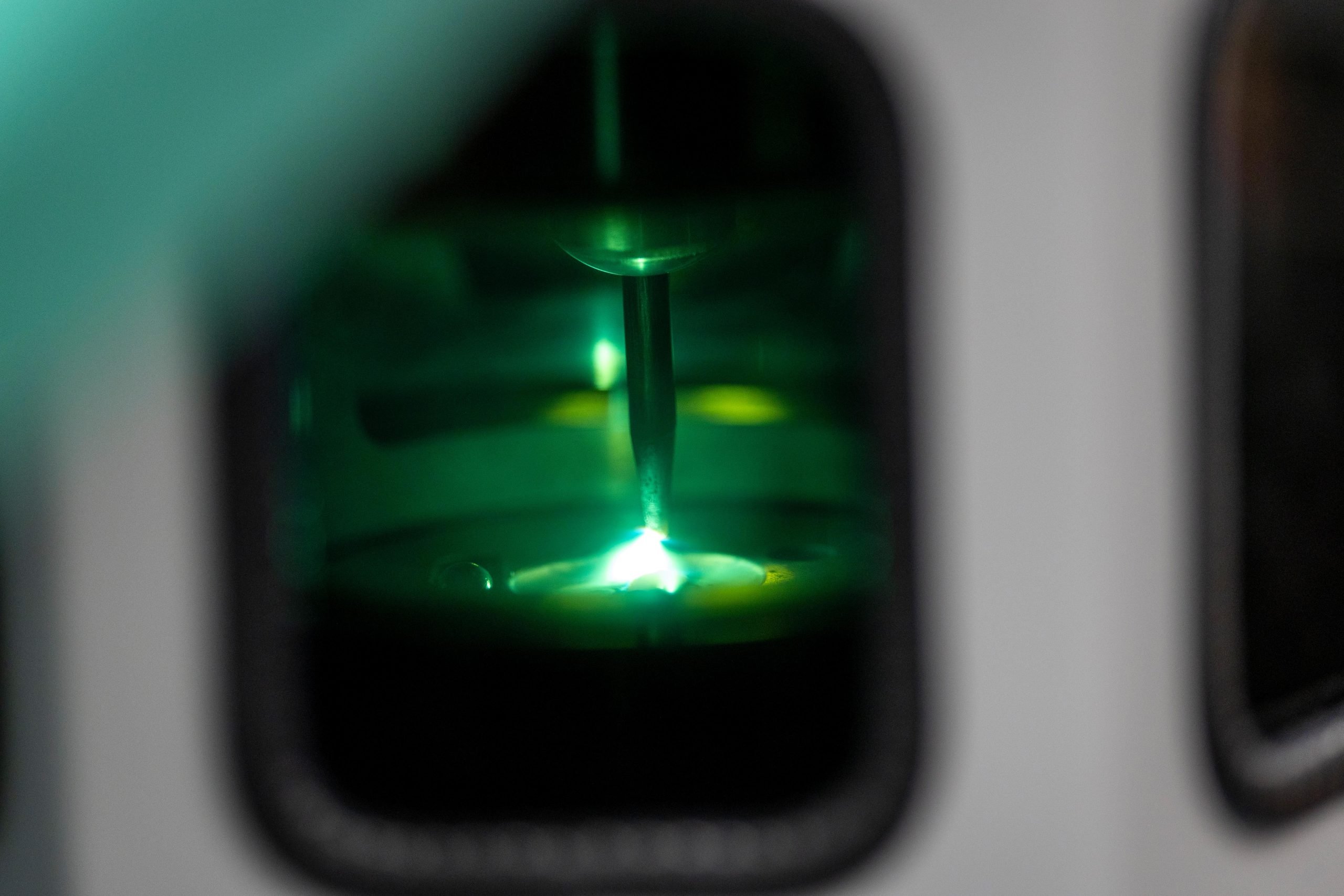
UAlbany chemists created manganese diboride, a high-energy material with potential for rocket fuel and new technologies.
Chemists at the University at Albany have developed a high-energy compound that could transform rocket fuel and make space travel more efficient. When ignited, this compound produces significantly more energy per unit of weight and volume than current propellants.
For rockets, this means that less fuel would be needed to achieve the same mission duration or payload capacity, leaving more space for essential equipment and supplies. The research was published in the Journal of the American Chemical Society.
“In rocket ships, space is at a premium,” said Assistant Professor of Chemistry Michael Yeung, whose lab led the work. “Every inch must be packed efficiently, and everything onboard needs to be as light as possible. Creating more efficient fuel using our new compound would mean less space is needed for fuel storage, freeing up room for equipment, including instruments used for research. On the return voyage, this could mean more space is available to bring samples home.”

The compound, manganese diboride (MnB2), is more than 20% higher in energy density by weight and about 150% higher by volume compared with aluminum, which is currently used in solid rocket boosters. Despite its potency, it is remarkably stable and only ignites when exposed to an ignition source such as kerosene.
Beyond rocket propulsion, the boron-based structure of MnB2 shows wide-ranging potential. Work from the Yeung lab indicates it could also strengthen catalytic converters in automobiles and act as a catalyst for breaking down plastics.
It Takes Heat to Make Heat
Manganese diboride is part of a group of chemical compounds long suspected to have unusual properties, but progress in studying them has been limited by the challenge of actually producing the material.
“Diborides first started getting attention in the 1960s,” said UAlbany PhD student Joseph Doane, who works with Yeung. “Since these initial looks, new technologies are allowing us to actually synthesize chemical compounds that were once only hypothesized to exist.

“Knowing what we do about the elements on the periodic table, we suspected that manganese diboride would be structurally asymmetrical and unstable — factors which together would make it highly energetic — but until recently, we couldn’t test it because it couldn’t be made. Successfully synthesizing pure manganese diboride is an exciting achievement in and of itself. And now, we can test it experimentally and discover new ways to put it to use.”
Producing manganese diboride requires extreme heat, generated by a device known as an “arc melter.” To begin, manganese and boron powders are pressed into a pellet and sealed inside a reinforced glass chamber. A narrow electrical current is then directed at the pellet, heating it to nearly 3,000°C (over 5,000°F). The molten substance is rapidly cooled to preserve its structure. On the atomic scale, this process forces the central manganese atom to bond with more atoms than usual, creating a crowded arrangement tightly compressed like a coiled spring.
Unlocking the structure through deformation
When exploring new chemical compounds, being able to physically produce the compound is critical. You also need to be able to define its molecular structure in order to better understand why it behaves the way it does.
UAlbany PhD student Gregory John, who works with computational chemist Alan Chen, built computer models to visualize manganese diboride’s molecular structure. These models revealed something critical: a subtle skew, known as “deformation,” which gives the compound its high potential energy.
“Our model of the manganese diboride compound looks like a cross-section of an ice cream sandwich, where the outer cookies are made of a lattice structure comprised of interlocking hexagons,” said John. “When you look closely, you can see that the hexagons aren’t perfectly symmetrical; they’re all a little skewed. This is what we call ‘deformation.’ By measuring the degree of deformation, we can use that measure as a proxy to determine the amount of energy stored in the material. That skew is where the energy is stored.”
Here’s another way to picture it.
“Imagine a flat trampoline; there’s no energy there when it’s flat,” said Yeung. “If I put a gigantic weight in the center of the trampoline, it will stretch. That stretch represents energy being stored by the trampoline, which it will release when the object is removed. When our compound ignites, it’s like removing the weight from the trampoline and the energy is released.”
New Materials Need New Compounds
“There’s this consensus among chemists that boron-based compounds should have unusual properties that make them behave unlike any other existing compounds,” said Associate Professor of Chemistry Alan Chen. “There’s an ongoing quest to figure out what those properties and behaviors are. This sort of pursuit is at the heart of materials chemistry, where creating harder, stronger more extreme materials requires forging brand-new chemicals. This is what the Yeung lab is doing — with findings that could improve rocket fuel, catalytic converters and even processes for recycling plastics.
“This study is also a great example of the scientific process, where researchers pursue interesting chemical properties even when they’re not certain what specific applications might emerge. Sometimes, present case included, the results are serendipitous.”

Yeung’s interest in boron compounds started when he was a grad student at the University of California, Los Angeles. His project aimed to discover compounds harder than diamond.
“I distinctly remember the first time I made a compound related to manganese diboride,” Yeung said. “There I was, holding this new material that was supposed to be super hard. Instead, it started to get hot and changed into a pretty orange color. I thought, ‘Why is it orange? Why is it glowing? It shouldn’t be glowing!’ That’s when I realized how energetic boron compounds can be. I put a pin in it to explore in the future, and that’s exactly what we are working on now.”
Reference: “Violations of Coordination: Exploring Metastable Diborides via Energetic Transition Metals” by Joseph T. Doane, Gregory M. John, Alma Kolakji, Abraham A. Rosenberg, Yiren Zhang, Alan A. Chen and Michael T. Yeung, 2 May 2025, Journal of the American Chemical Society.
DOI: 10.1021/jacs.5c04066
Never miss a breakthrough: Join the SciTechDaily newsletter.