Astronomers using the Hubble Space Telescope have identified an entirely new kind of object in the Universe.
This strange object is a starless, gas-rich cloud that’s dominated by dark matter, and is thought to be a remnant left over from the…
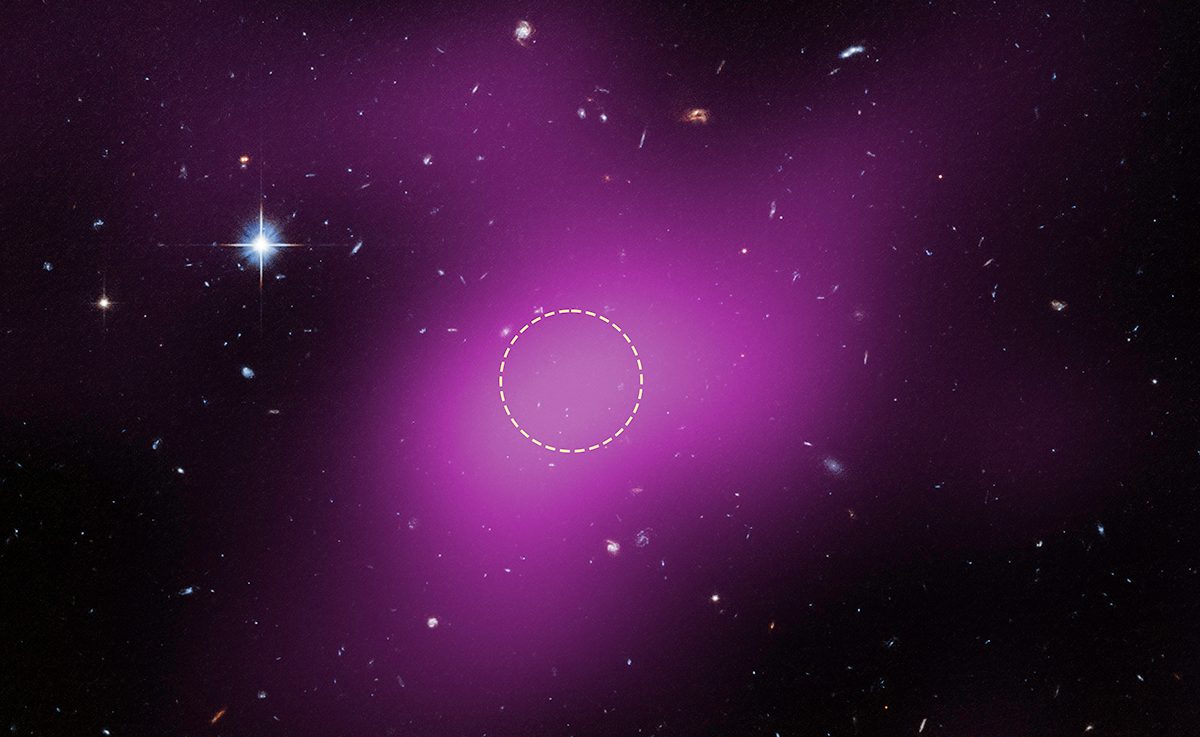
Astronomers using the Hubble Space Telescope have identified an entirely new kind of object in the Universe.
This strange object is a starless, gas-rich cloud that’s dominated by dark matter, and is thought to be a remnant left over from the…