REAL-WORLD evidence from Denmark indicates that biologic drug survival in psoriasis differs markedly depending on whether patients are biologic-naive or have prior biologic exposure. The findings are based on nationwide registry data and…
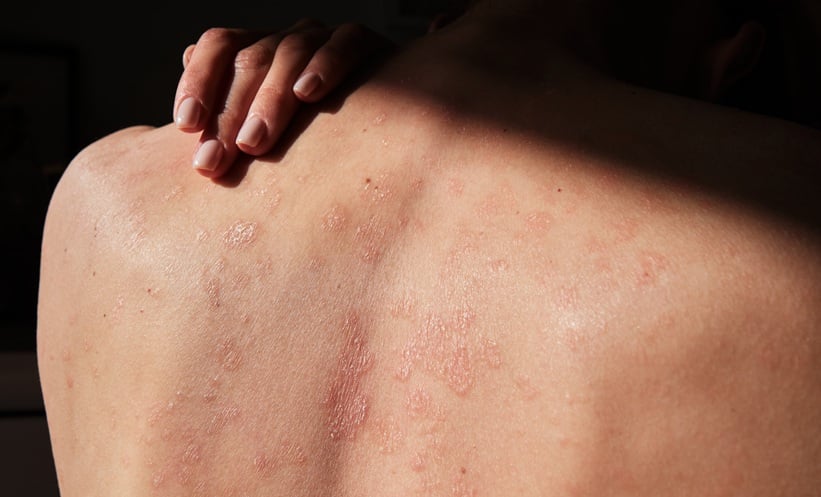
REAL-WORLD evidence from Denmark indicates that biologic drug survival in psoriasis differs markedly depending on whether patients are biologic-naive or have prior biologic exposure. The findings are based on nationwide registry data and…