The chemical mood of Earth’s mantle, its redox state, is shaped by how iron and carbon behave as they gain or lose electrons. This redox balance affects everything, from the formation of minerals to the movement of heat and materials deep underground.
Studies of mantle rocks (xenoliths), laboratory experiments, and thermodynamic models indicate that oxygen levels decrease with depth, particularly down to approximately 250 km. Around 250–300 km, scientists expect a nickel-rich metallic alloy to form, resulting in a further decrease in oxygen.
But here’s the twist: garnets found between 250 and 500 km tell a different story. They exhibit signs of more oxidized conditions, and no nickel-rich alloy has ever been seen in these depths to support the prediction.
Credit
Yaakov Weiss
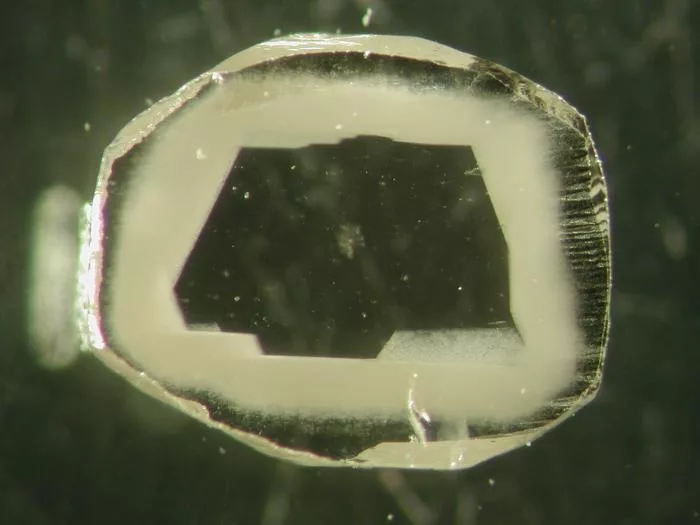
A groundbreaking study in Nature Geoscience, led by researchers at the Hebrew University of Jerusalem, has uncovered tiny metallic and carbonate inclusions inside diamonds from South Africa’s Voorspoed mine, offering a rare peek into Earth’s deep mantle chemistry.
Are there lost worlds in Earth’s interior?
Working with scientists from the University of Nevada, the University of Cambridge, and the Nanocenter at Hebrew University, the team found nickel–iron nanoinclusions and nickel-rich carbonate microinclusions in diamonds formed 280–470 km beneath Earth’s surface. These microscopic features provide the first direct evidence of nickel-rich alloys at the depths where they were long predicted to exist.
This discovery validates key mantle redox models and helps explain how iron and nickel behave deep underground, shedding light on the chemical forces that shape our planet from within.


Credit
Yaakov Weiss
The diamonds from South Africa’s Voorspoed mine carry more than sparkle; they hold clues to Earth’s deep interior. Their mineral cargo includes coesite, potassium-rich aluminous phases, and solid nitrogen, all of which act as pressure markers that pinpoint their origin to the deep upper mantle and the shallow transition zone.
But the real breakthrough goes beyond confirming theory. The presence of both nickel–iron alloy and nickel-rich carbonate inside the diamonds suggests a dramatic underground event: a metasomatic redox-freezing reaction. In this process, an oxidized, carbon-rich melt infiltrates reduced, metal-bearing peridotite, triggering the formation of diamonds. This supports earlier findings from shallower depths, reinforcing the idea that such redox reactions are a key pathway for the creation of natural diamonds.
Potential ‘diamond factory’ may have existed at the core-mantle boundary for billions of years
In this deep mantle setting, iron oxidized more readily than nickel, leaving behind a nickel-rich metallic alloy. Meanwhile, nickel-rich carbonates and diamonds began to crystallize from the surrounding melt. The diamonds, in essence, captured a brief but powerful geochemical transformation, where reduced mantle rock was turned into a more oxidized, volatile-rich zone, and carbonates were reduced to form diamonds.
“This is a rare snapshot of mantle chemistry in action,” says Weiss. “The diamonds act as tiny time capsules, preserving a reaction that would otherwise vanish as minerals re-equilibrate with their surroundings.”


Credit
Yaakov Weiss
Their microscopic inclusions suggest that localized redox reactions in the mantle can oxidize small pockets of rock. This may explain why some superdeep diamonds show unexpectedly high oxidation levels.
These reactions also help us understand how volatile-rich magmas form. When mantle peridotite is enriched with carbonate, potassium, and other elements during redox events, it sets the stage for explosive magmas like kimberlites and lamprophyres, and even some ocean island basalts. In short, these tiny mineral traces reveal sweeping connections between subduction, mantle chemistry, and the fiery forces that shape continents and deliver diamonds to the surface.
Unraveling secrets of diamond formation
The study underscores the scientific value of diamonds as more than just gemstones. Their inclusions, whether nanometer-scale alloys or high-pressure minerals, offer one of the only natural records of conditions hundreds of kilometers beneath our feet.
Kempe and Weiss’s research marks a breakthrough, the first natural evidence of nickel-rich alloys at the depths long predicted by mantle models. These tiny inclusions within diamonds provide a vivid snapshot of how Earth’s deep redox landscape evolves through melt-rock interactions.


Credit
Yaakov Weiss
Far from being static symbols of permanence, diamonds are emerging as storytellers of transformation. Locked within their crystal structure are clues to the mantle’s hidden chemistry, records of oxidation, volatile enrichment, and dynamic processes that continue to shape our planet from within. As scientists delve deeper into these mineral time capsules, they’re uncovering a rich narrative of Earth’s evolving interior.
Journal Reference:
- Kempe, Y., Remennik, S., Tschauner, O. et al. Redox state of the deep upper mantle recorded by nickel-rich diamond inclusions. Nat. Geosci. (2025). DOI: 10.1038/s41561-025-01791-4
