Attached to many proteins are glycans—complex strings of sugars—that change how the whole unit functions. Removing them makes it easier to study both the proteins and glycans. But many glycans don’t have a matching enzyme that can efficiently cleave them. This is especially true for O-glycans, which are attached to up to 83% of proteins secreted by cells.
A new study led by Stephen Withers from the University of British Columbia introduces a technique for engineering much more efficient enzymes. He and his team created a genetically modified enzyme with an 840-fold improvement over the natural enzyme in cleaving an O-glycan (ACS Cent. Sci. 2025, DOI: 10.1021/acscentsci.5c01227).
To create an enzyme that cleaves the O-glycan sialyl T-antigen (STAg), Withers’s team genetically modified an existing enzyme that naturally has a small amount of STAg-cleaving activity. The researchers modified it by introducing random mutations into the genes encoding that enzyme in Escherichia coli host cells and also by causing mutations in the DNA responsible for the enzyme’s active binding site. Through this directed evolution process, they created tens of thousands of E. coli cells—and hoped that one would contain an enzyme that would cleave the glycan efficiently.
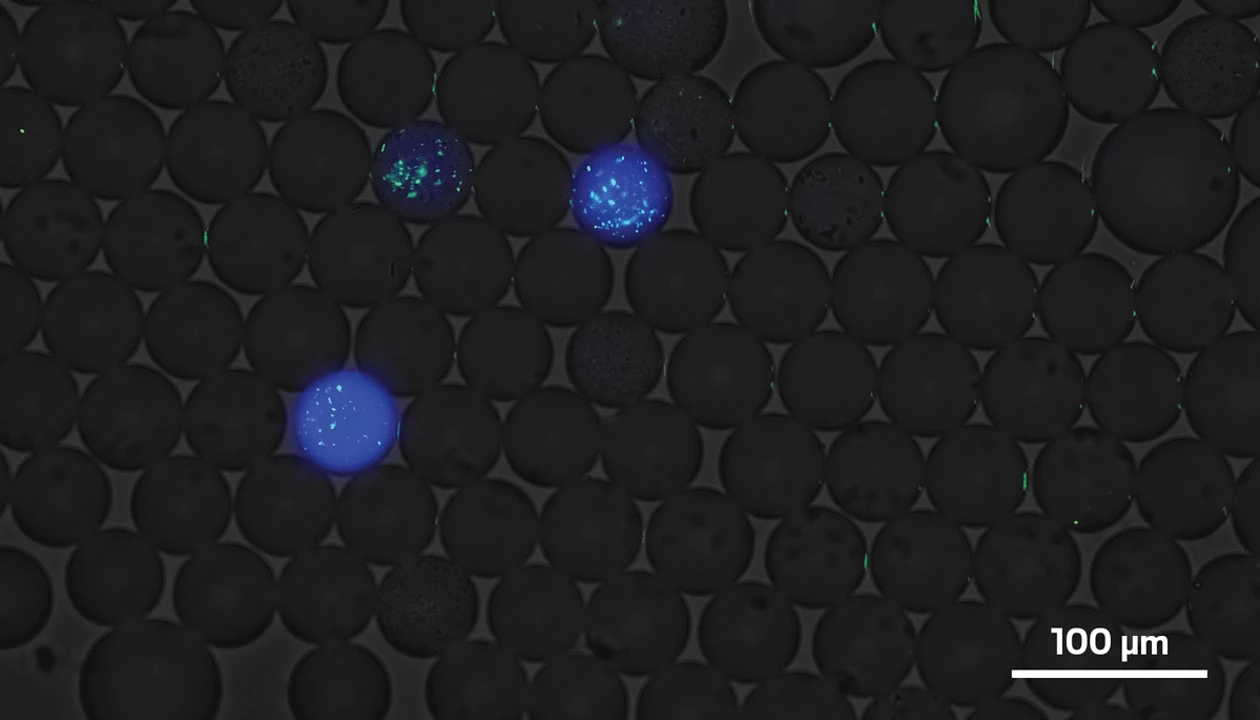
To screen this library of enzymes, the team suspended tens of thousands of oil droplets in water, each of which contained one E. coli cell. The researchers mixed in a protein with both a STAg glycan attached to it and DNA that would cause blue fluorescence when the glycan was removed. After a few days, they measured enzyme activity by observing how much each droplet glowed.
Most of this is standard protocol. What Withers’s team did differently was add DNA to the enzyme to make it fluoresce green. A brighter glow from the enzyme meant there was more of it. By comparing both colors of light, the researchers were able to compare the amount of each enzyme to the level of successful cleaving activity. This comparison allowed them to identify enzymes that are great at cleaving STAg but aren’t expressed in high numbers by E. coli. Without this step, they might have overlooked potent enzymes. (Later, the team will be able to edit E. coli to better express the best enzymes.)
“The methodology they’ve developed is really unique. I’ve never seen anything like it,” says Stacy Malaker, a chemist at the Yale School of Medicine who was not involved in the study.
Using this technique, Withers’s team found an enzyme that cleaves STAg 840 times as effectively as the original unmodified enzyme—far better than the researchers expected. “Quite often, you only end up with a 5- or 10-fold increase, and that would make a good paper,” Withers says. He credits the new method for the improvement because other techniques he’s tried have not improved enzymes to anywhere near this degree.
“This lays the groundwork for developing other enzymes that can remove biologically relevant or disease-specific O-glycans,” Malaker says. It also has potential therapeutic applications—for example, removing glycans found only on tumor cells, she says.
The process could be broadened beyond enzymes that cleave glycans, Withers adds. So long as scientists can make an enzyme fluoresce and synthesize a substrate that glows after the reaction, they can use it to optimize any enzyme, he says.
Chemical & Engineering News
ISSN 0009-2347
Copyright ©
2025 American Chemical Society