How does a single nutrient shape early development? Omega-3 fatty acids, especially docosahexaenoic acid, or DHA, are essential for building healthy brains and eyes. However, scientists still don’t fully understand how DHA works at the cellular level. Now, using a transparent aquatic embryo and some genetic editing tools, researchers are illuminating DHA’s role in development.
In a recent paper in the Journal of Lipid Research, researchers from the University of Colorado Anschutz developed a zebrafish model to study the impacts of DHA-deficiency during embryonic development.
Katie Ranard
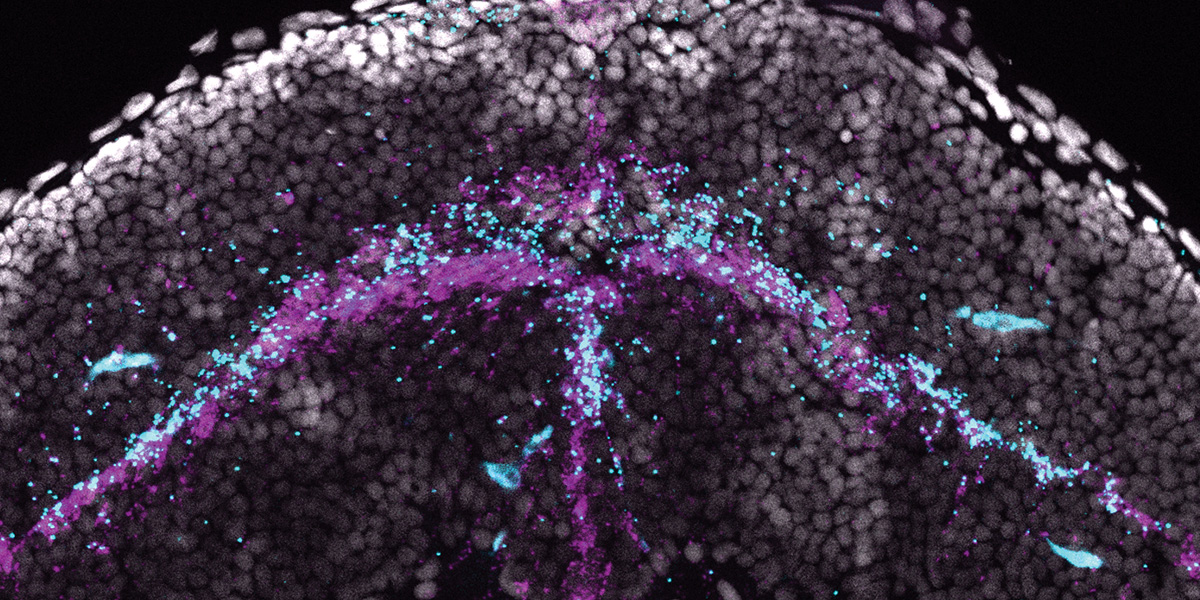
Docosahexaenoic acid, or DHA, which is crucial for vision and brain development, is primarily obtained through the diet, but it can also be synthesized in the body. Shown here is a florescence microscopy image of a developing zebrafish larva expressing a DHA biosynthesis gene called elovl2 in the brain (cyan). Elovl2 expression also overlaps with a gene marking astrocytes (pink) within the brain (blue).
During pregnancy, omega-3 nutrition becomes even more important. The developing fetus relies entirely on maternal nutrients, particularly for the growth of the nervous system. Katie Ranard, a postdoctoral fellow in the lab of Bruce Appel, a professor of pediatrics and developmental biology, has been motivated to study the role of nutrition in embryonic development since her Ph.D., where she investigated the molecular effects of nutrients in the mouse brain.
“I knew I wanted to dig deeper into how nutrients impact the cellular/molecular mechanisms guiding brain development,” Ranard said. “We set out to create a new type of animal model that would allow us to study fundamental micro-level (nutritional) factors during neurodevelopment, (which) could help guide nutritional recommendations and interventions for pregnant women and ultimately improve the health of future generations.”
To investigate DHA’s role in early development, researchers turned to zebrafish. These embryos are ideal for this type of research because they develop outside the body, grow quickly and remain transparent, allowing scientists to directly observe development in real time.
The authors modified zebrafish embryos using a genetic approach that focused on the elongation of very long-chain fatty acids protein 2, or elovl2, which plays a rate-limiting role in the biosynthesis pathway of DHA.
Using CRISPR/Cas9 technology, they altered the elovl2 gene, generating mutant embryos. The team then combined this with a diet lacking DHA to create a robust model of DHA deficiency.
When characterizing the novel DHA-deficient model, Ranard said she observed that some offspring developed abnormally small eyes, while others had normal-looking eyes but showed dysregulated vision and immune system genes. These results align with previous studies linking low DHA to impaired immunity.
While much of elovl2 research is focused on the eye, this protein is also expressed broadly across the central nervous system. Ranard said future work will use this model to study DHA and neurodevelopment more broadly.
“Our next step is to use our model to study whether low DHA status leads to the abnormal development/behavior of specific brain cell types,” Ranard said. “(This) will provide a unique window into how DHA status affects neurodevelopment at the cellular level.”
This multidisciplinary study bridges nutrition and developmental neurobiology, leading to a new way to study maternal nutrition.
“My hope is that our zebrafish model will open new doors for how we, as a research community, investigate nutrition’s role during neurodevelopment,” Ranard said. “I believe it’s important that we continue to think outside of the box and adopt new techniques and approaches.”