Reactions that sever typically unreactive carbon-hydrogen bonds, known as C–H activations, are a well-studied staple of organic chemistry. So it’s a well-known fact that palladium-based catalysts tend to work better than ones based on palladium’s cheaper, possibly greener first-row counterpart, nickel.
But among the reams of scholarly papers about C–H activation methods and mechanisms, Demyan Prokopchuk saw something missing: a detailed comparison of nickel and palladium’s C–H bond-breaking abilities under identical conditions.
“Really, nobody took a step back and asked, ‘How do we systematically even measure the C-H bond strength in this activation step?’ ” he says. He and his team at Rutgers University–Newark took it upon themselves to change that.
The plan was simple, as these things go: create model complexes—one nickel based, the other palladium based, but otherwise identical—in which the metal center coordinates to a carbon-hydrogen bond in a carefully chosen alkane pincer ligand. The next step was to experimentally determine using nuclear magnetic resonance spectroscopy and acid-base equilibria how much each metal weakens, or activates, the bond.
Prokopchuk and his group published their initial results on the nickel complex in 2022 (J. Am. Chem. Soc. DOI: 10.1021/jacs.2c05667). Their investigation into the palladium complex came out on Sept. 9 (J. Am. Chem. Soc. 2025, DOI: 10.1021/jacs.5c07649).
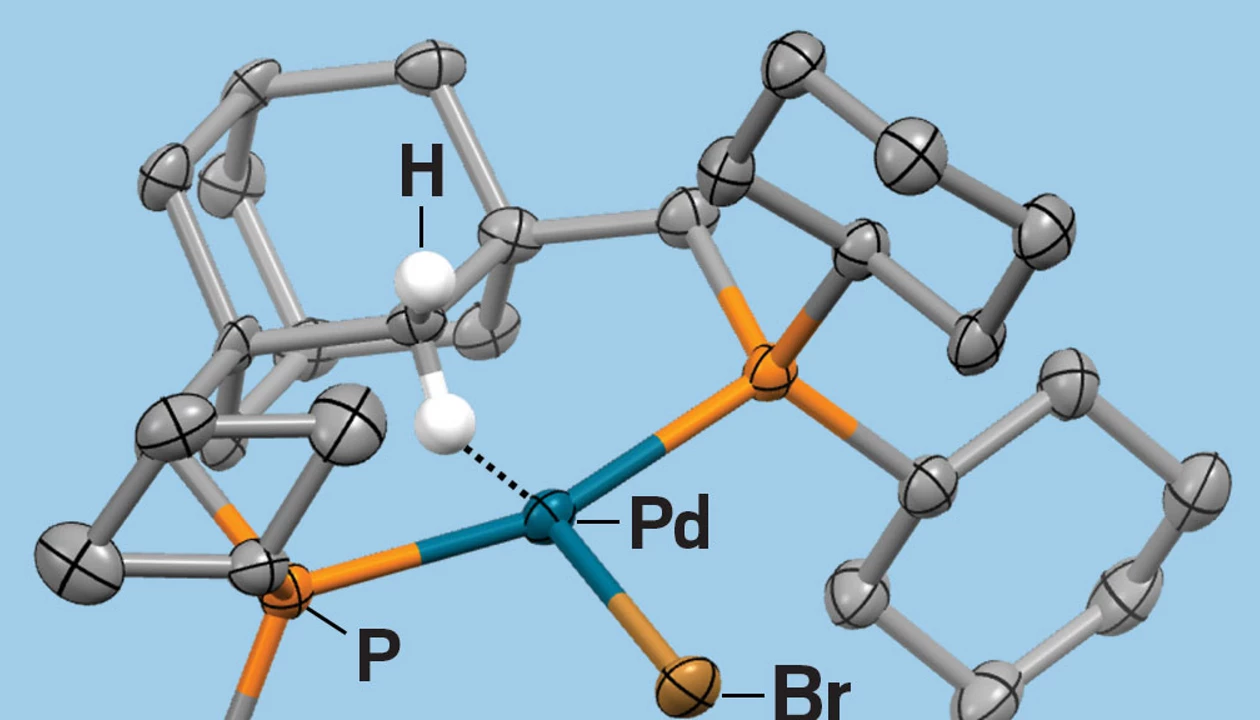
Synthesizing the complexes was fairly straightforward, Prokopchuk says. By using X-ray crystallography, the researchers got a good “solid-state snapshot,” he says, of a key intermediate containing an agostic interaction, meaning the hydrogen atom is bound partly to carbon and partly to the metal center.
Measuring the acid-base equilibrium turned out to be harder for palladium than nickel because palladium had a tendency to reversibly form bimetallic dimers. It took a couple of years to figure out what was going on and how to disentangle the data to measure the proton transfer equilibrium the researchers were looking for.
“This is interesting, but also often complicated chemistry,” says Andreas Hansen of the University of Bonn, whose group handled the computational aspects of the study. There was a lot for both the experimentalists and theorists to sink their teeth into in the project.
Ultimately, the researchers found that the palladium complex renders the C–H bond around 100,000 times as acidic (read: willing to part with H+) as it is in the nickel complex.
This not only gives quantitative weight to empirical observations of how palladium and nickel behave but may also help chemists figure out how to optimize their catalytic systems. For example, it suggests that nickel catalysts would benefit from being paired with stronger bases.
Tianning Diao, an organic chemist at New York University who studies C–H activation but was not involved with this work, describes the paper in an email to C&EN as “remarkable in many respects.” The synthesis and characterization of the palladium complex show “outstanding creativity in molecular design” and accompany a thorough mechanistic study that “provides long-sought experimental evidence for a hypothesis that has been speculated on” in the C–H activation community.
Prokopchuk says his team’s next steps are to continue with more head-to-head comparisons of other catalyst metals, such as cobalt, iridium, and rhodium.
Chemical & Engineering News
ISSN 0009-2347
Copyright ©
2025 American Chemical Society