Formulation of lipid nanoparticles
Lipid nanoparticles were formulated following the Onpattro formulation and using a model oligonucleotide, siHPRT, as a cargo. Details regarding LNP formulation, mixing, and downstream processing are provided in the materials and methods. Briefly, a four-component lipid mixture was solubilized in ethanol to a total lipid concentration of 12.5 mM (MC3: DMG-PEG-2000: DSPC: cholesterol at a molar ratio of 50: 1.5: 10: 38.5). siHPRT was solubilized in 50 mM sodium citrate, pH 5, at an N:P ratio of 6. The ethanol and aqueous solutions were then mixed via a T-junction mixer at a flow rate ratio of 3:1 (aqueous:ethanol, vol%) (Supplementary Fig. 1). After further dilution and a brief hold, siRNA-LNP solutions were then concentrated to an siRNA concentration of ~0.4–0.5 mg/mL and buffer exchanged into the appropriate storage buffer. Buffer exchange was performed primarily through tangential flow filtration (TFF), but was completed using desalting columns at small scales in some instances. After buffer exchange and prior to long-term storage, all siRNA-LNP particle sizes were 70 ± 5 nm with polydispersity indexes ≤0.2 and encapsulation efficiencies >85%.
Colloidal stability of LNPs within phosphate buffer
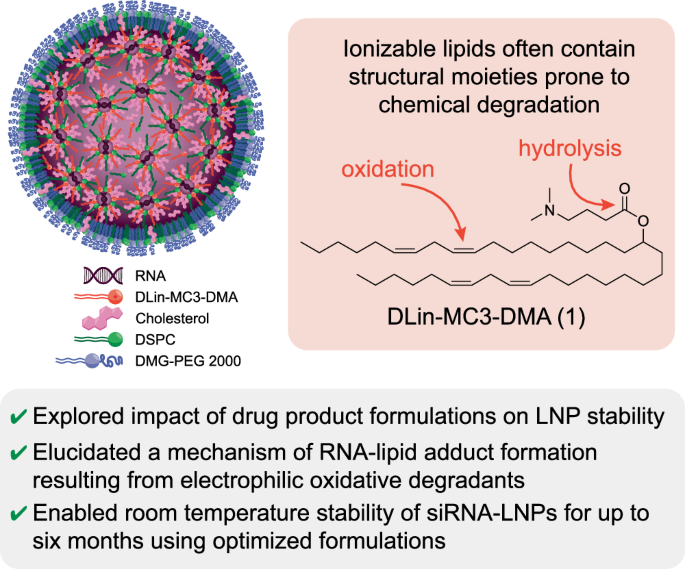
To explore the impact of drug product matrix on the siRNA-LNP formulations, we initially studied 1X PBS, pH 7.4 (without calcium or magnesium). This formulation was selected because siRNA-LNPs can be kept refrigerated for long-term stability, and it is expected to closely mirror the formulation of Onpattro3. At the initial time point, the transparency of siRNA-LNP solutions was dependent on particle concentration, but generally was a homogenous off-white solution (Fig. 2A). Particle size was corroborated by cryoEM analysis, which confirmed a spherical morphology with a uniform, electron-dense core with few phase-separated blebs (Fig. 2B)13,14,15. Vials were then boxed to minimize exposure to light and stored either at refrigerated conditions (2–8 °C) or at room temperature (RT, ~22–25 °C) over a period of up to six months. After a minimum storage time of one month, visual inspection of vials stored at RT showed early signs of particle aggregation and by two and three months, phase separation was apparent (Fig. 2A, with phase separation denoted by red arrows). We visually observed that aggregation began as small, dispersed particulates (1 month), accumulated at the air-water interface as a white film (2 and 3 months), then largely sedimented to the bottom of the vial as a white, wispy aggregate (6 months). By comparison, vials at 2–8 °C remained homogenous. In addition to macroscopic growth, dynamic light scattering analysis (DLS) showed that particle hydrodynamic diameter increased from ~73 to 127 nm and polydispersity index (PDI) increased to ≥0.30 (Fig. 2C, D). Intensity-based scattering exhibited multimodal particle size distributions (Supplementary Fig. 2), with populations ranging from 70–200 nm as well as >5 µm. To capture count rates of sub-visible particles, Micro-Flow Imaging (MFI) was performed. Particle aggregation resulted in (i) higher particle/mL count rates (1–100 µm) and (ii) a shift of macroscopic particle distributions to larger diameters (Supplementary Fig. 3). Nanoparticle tracking analysis of siRNA-LNPs held at either 5 or 40 °C over one week demonstrated a statistically significant decrease in sub-micron particles, particularly those around ~70 nm, for samples at elevated temperatures (Supplementary Fig. 4). Collectively, these data corroborate that smaller particles gradually aggregate at higher temperatures to form larger, micron-sized aggregates in phosphate-based buffers. By comparison, when stored refrigerated, mean particle sizes remained below 100 nm and mean PDIs remained below 0.2.
A Compilation of LNP samples photographed upon removal from storage at 2–8 °C or 25 °C for up to six months. Red arrows denote where phase separation is occurring. B Visualization of an LNP sample stored at room temperature for four weeks then visualized by cryogenic electron microscopy (scale bar = 100 nm). A portion of the image is magnified in the inset. LNP diameters were individually measured (n = 326) using image processing software, and a histogram (bin width = 2.5 nm) of relative frequency was constructed alongside a curve resulting from nonlinear regression on the data. The impact of LNP storage in PBS at 2–8 °C or 25 °C was compared to defined thresholds for the following critical quality attributes: C Z-average particle diameter and D polydispersity index by dynamic light scattering, E encapsulation efficiency by RiboGreen assay, and amounts of F intact MC3 1, G intact siHPRT antisense strand, and H intact siHPRT sense strand by LC/MS. Bar height and error bars represent the mean and standard deviation of four biological replicates, all of which are independently plotted as closed squares. Encapsulation efficiencies over time at 5 and 25 °C (E) were evaluated by linear regression analysis and had two-tailed ANOVA p-values of 0.12 and 0.45, respectively. The portion of a graph highlighted in green represents the values that meet the threshold set for the critical quality attribute. Source data are provided as a Source Data file.
Stability of ionizable lipid and encapsulated siRNA cargo
Next, we investigated the ability of siRNA to leach from the LNP vehicles over time. RiboGreen assays were performed and revealed that encapsulation efficiencies (EE%) were similar over time regardless of storage temperature (Figs. 2E, 5 and 25 °C had p-values of 0.12 and 0.45, respectively). By the same analysis, the siRNA content remained similar in all samples over time (Supplementary Fig. 5, 5 and 25 °C had p-values of 0.38 and 0.85, respectively). In addition to colloidal stability, we sought to investigate the integrity of both the ionizable lipid as well as the siRNA cargo through liquid chromatography mass spectrometry (LC/MS) analysis. We first evaluated the stability of MC3 lipid itself within 1X PBS at 5, 25 and 40 °C for upwards of four weeks (Supplementary Fig. 6). Surprisingly, rapid degradation of the lipid was observed, with >25% degradation occurring within four weeks at 5 °C, or at one week for both 25 °C and 40 °C (Supplementary Fig. 6A). The major degradation pathway was oxidation, with oxidative byproducts representing 25–30% of the degraded lipid (Supplementary Fig. 6A–C). Hydrolysis byproducts were observed but in lower amounts, but negligible in most instances (Supplementary Fig. 6A–C).
We then analyzed MC3 integrity when the lipid was formulated within siRNA-LNPs. During storage over six months, MC3 lipid integrity dropped by ~5% and ~9% at 2–8 °C or RT, respectively (Fig. 2F). Byproducts were identified and are noted within Supplementary Table 1 and Supplementary Fig. 19, which revealed that most impurities come from tail oxidation. Other byproducts, like those associated with ester hydrolysis or head group oxidation, were observed but in comparatively lower amounts. Surprisingly, many of these oxidized impurities are present even at the initial time point, indicating likely degradation of the lipid prior to particle fabrication. Overall, while the degradation of MC3 appears to be slower within nanoparticle systems than when solubilized within ethanolic PBS, it is still not avoided. Whether this slowed degradation is a result of lipids being packed within a nanoparticle or due to downstream removal of residual impurities from the LNP material (e.g., TFF) is a focus of ongoing investigation. These results corroborate the precautions necessary for handling sensitive lipids like MC3 at the manufacturing scale3, though it is worth highlighting that many researchers may not go to these same lengths at the discovery stage.
After confirming the oxidation of MC3 within RNA-LNPs, we were curious to examine the integrity of the encapsulated siRNA payload. Previous reports have noted how oxidative lipid impurities may concurrently cause the oxidation of encapsulated mRNA1. Analysis of both the sense and antisense strand of the siRNA cargo revealed considerable degradation over time (Fig. 2G, H), with the level of intact sense and antisense strands falling to only 45% and 23%, respectively. Most of the degradation observed was a result of one or multiple conversions of phosphorothioates to phosphodiesters (PS-to-PO) (Supplementary Table 2). PS modifications have been identified in RNA and DNA16, and are commonly introduced into synthetic siRNA, ASO, and microRNA therapeutics to increase serum stability, cellular permeability, and plasma protein binding17,18,19, although PS modifications are less commonly explored for mRNA20,21. Here, we observe that PS motifs can be oxidized back to PO linkages22. Granted that these siRNA-LNPs have a high encapsulation efficiency and there is precedent suggesting ionizable lipids and analogous RNA cargoes tend to organize within the LNP core23,24,25, we speculate that the oxidation of these two molecules also occurs within the core (as opposed to within bulk solution). These results also highlight limitations of using the RiboGreen assay that other researchers have pointed out, namely that it is not an appropriate method of detecting RNA degradation or functionality26. Collectively, these data demonstrate the colloidal and chemical stability issues of siRNA-LNPs, including increases in particle size and polydispersity, microparticle aggregation, and oxidation of lipids and encapsulated siRNA.
Improving siRNA-LNP stability through drug product matrix optimization
We reflected on the stability issues of pharmaceutical excipients like polysorbate 80 that are known to undergo oxidation at the fatty acid chain27,28. In previous work, we studied the mechanism of polysorbate 80 oxidation and how buffer agents can impact degradation. In particular, we employed histidine-containing buffers that can mitigate excipient oxidation compared to phosphate buffers under light exposure and elevated temperatures29. Given the analogous oxidation problems observed with MC3, we evaluated the stability of siRNA-LNP systems within a 10 mM histidine buffer at pH 6.0. To match the osmolarity of 1X PBS, 140 mM sodium chloride was also included. At the initial time point, siRNA-LNPs in histidine-containing buffers were visually comparable to those formulated in PBS (Fig. 3A), had similar sizes (Fig. 3B, C, p-value of 0.96) and more narrow polydispersities (Fig. 3D, p-value of 0.02), as corroborated by cryoEM analysis. Interestingly, we noted that the nanoparticle morphology of siRNA-LNPs in histidine buffer had more blebs (Fig. 3B) than those in PBS, presumably due to variations in formulation pH. Compared to the visible aggregation within siRNA-LNPs in PBS, those formulated in histidine were consistently translucent, even after six months at RT (Fig. 3A). Over six months in 2–8 °C storage, there were no pronounced changes in size, PDI, or EE% (Fig. 3C–E). More strikingly, the siRNA-LNPs have remarkable colloidal stability even at RT storage, increasing by only ~11 nm over six months (73 to 84 nm, intensity data in Supplementary Fig. 7) with no notable changes in PDI (0.05 to 0.04), siRNA content (0.37 to 0.42 mg/mL) or EE% (97% to 98%). The amount of intact MC3 lipid decreased slightly over the six months with negligible differences between RT and 2–8 °C storage (94.0% and 92.0%, respectively).
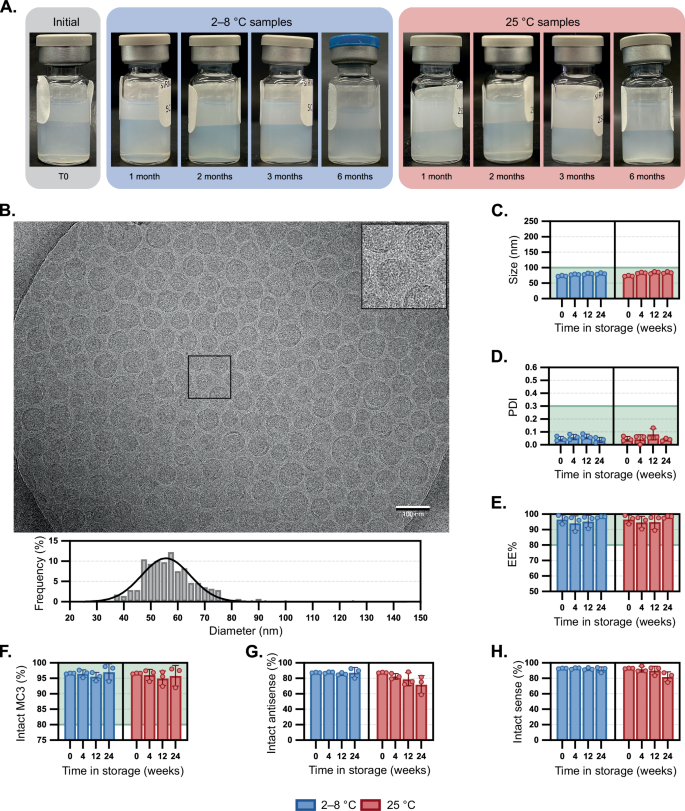
A Compilation of LNP samples photographed upon removal from storage at 2–8 °C or 25 °C for up to six months. B Visualization of a LNP sample stored at room temperature for four weeks then visualized by cryogenic electron microscopy (scale bar = 100 nm). A portion of the image is magnified in the inset. LNP diameters were individually measured (n = 277) using image processing software, and a histogram (bin width = 2.5 nm) of relative frequency was constructed alongside a curve resulting from nonlinear regression on the data. The mean diameter and standard deviation are listed. The impact of LNP storage in histidine at 2–8 °C or 25 °C was compared to defined thresholds for the following critical quality attributes: C Z-average particle diameter and D polydispersity index by dynamic light scattering, E encapsulation efficiency by RiboGreen assay, and amounts of F intact MC3 1, G intact siHPRT antisense strand, and H intact siHPRT sense strand by LC/MS. Bar height and error bars represent the mean and standard deviation of three biological replicates, all of which are independently plotted as closed squares. Particle sizes (C) and PDIs (D) at the initial time points were compared to those stored in PBS (Fig. 2C, D) via a two-tailed Student’s t-test assuming an unequal sample variance and had p-values of 0.96 and 0.02, respectively. The portion of a graph highlighted in green represents the values that meet the threshold set for the critical quality attribute. Source data are provided as a Source Data file.
Analysis of degradants suggested that while oxidation was still the primary driver of MC3 degradation, histidine largely mitigated it. Unfortunately, histidine was unable to entirely protect the siRNA cargo, which still experienced PS-PO conversion (Fig. 3G, H). These findings corroborate prior work employing antioxidants in dermatological formulations to mitigate desulfurization of PS-containing oligonucleotides30. Although significantly improved when compared to PBS formulation, there is room for improvement: for example, by screening other excipients, or increasing excipient concentrations (e.g., >20 mM). Similar stability profiles between MC3 and siRNA cargo suggest that the degradation mechanisms are correlated (Supplementary Fig. 8), supporting the notion that degradation of multi-component nanoparticle formulations is a complicated and oftentimes intertwined process.
To demonstrate the generalizability of the histidine buffer platform, we systematically explored three additional cationic/ionizable lipids: DOTAP, DODMA, and DLin-KC2-DMA. We formulated siHPRT-LNPs with each lipid and buffer-exchanged the resulting particles into either PBS or the histidine-NaCl buffer. To accelerate degradation, select samples were spiked with 1 ppm of either hydrogen peroxide or metals (nickel, iron, and copper). Particles were then stored at either 25 or 40 °C for up to four weeks, and analyzed for changes in colloidal stability, siRNA payload integrity, and ionizable lipid integrity. Starting with DOTAP, we observed changes in siRNA-LNP stability for particles stored in PBS, including particle size, payload, and ionizable lipid integrity (Supplementary Fig. 9). In all instances, degradation could be mitigated by using histidine buffer. Notably, DOTAP lipid was more sensitive to hydrolysis than oxidation, which we hypothesized was due to there being a single alkene in each lipid tail and two ester linkages (Supplementary Table 3). To confirm this, we next leveraged a DODMA ionizable lipid that contains a single alkene in each lipid tail but lacks esters (Supplementary Fig. 10). Accordingly, DODMA-LNPs proved more resilient to colloidal instability and ionizable lipid degradation (Supplementary Table 4); however, payload integrity continued to prove problematic for particles stored in PBS spiked with either metals or peroxides, whereas those stored in histidine retained payload integrity across all conditions. Finally, DLin-KC2-DMA, a lipid which preceded the optimization of MC3, was employed due to its lack of esters (instead containing a cyclic acetal linker) and structurally similar bis-allylic dilinoleyl tail (Supplementary Fig. 11). DLin-KC2-DMA LNPs demonstrated dramatic colloidal and chemical degradation when stored in PBS, whereas histidine buffers again mitigated these changes over time (Supplementary Table 5); however, histidine buffers were unable to rescue samples spiked with metals. The increased sensitivity of MC3 and DLin-KC2-DMA to oxidation in comparison to DOTAP and DODMA is supported by the fact that lipids with higher degrees of unsaturation oxidize more rapidly due to the weakness of a bisallylic C–H bond compared to an allylic C–H bond31. For example, linoleic acid is known to oxidize faster than oleic acid32. However, while each cationic/ionizable lipid may differ in its particular degradation mechanisms, the ability of histidine buffers to improve product stability is shown to be generalizable across this class of LNPs. To our knowledge, this is the first demonstration of histidine-containing formulations showing such a dramatic improvement on siRNA-LNP room temperature stability.
Oxidative degradants of unsaturated ionizable lipid tails may react with siRNA cargo
While mass spectrometry was useful in identifying the molecular weight of degradants, we moved to NMR for further structure identification via a combination of 1H, HSQC, COSY, and HMBC experiments. The elucidation of MC3’s oxidative impurities has been the focus of previous reports and innovative methods, which have suggested oxidation of the lipid tail via epoxide formation6. We sourced MC3 from three vendors and analyzed the raw material by two-dimensional 1H-NMR. For all lots, two primary degradants were identified, both oxidative impurities in the lipid tail corresponding to either dienone (major impurity, 2, Fig. 4A) or dienol species (minor impurity, S1, Supplementary Fig. 12). These impurities include those previously identified for oxidation of linoleic acid, though the ketone is less commonly reported (note: here the ketone is drawn internal (towards head), though it could also be terminal (towards tail))33,34. Density functional theory (DFT) calculations were employed to corroborate the oxidative instability of the bis-allylic C-H bond in the MC3 1 tail and to help explain the dienone 2 and dienol S1 oxidative byproducts, with the mechanism supplied in Supplementary Fig. 13. To our knowledge, this is the first time that byproducts 2 and S1 have been identified for MC3, despite its widespread use as an ionizable lipid and known oxidation challenges.
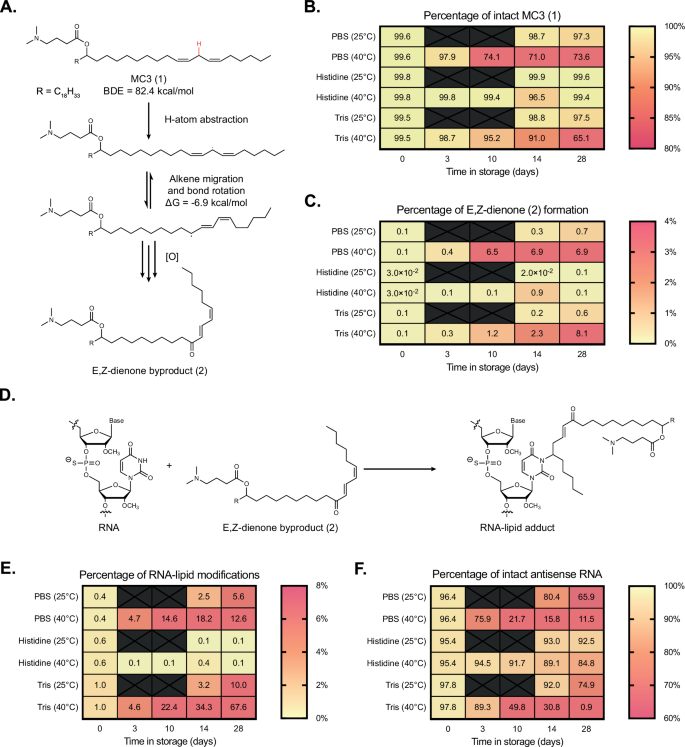
A Proposed mechanism of MC3 1 oxidation to form E,Z-dienone byproduct 2. Full mechanism supplied in Supplementary Fig. 10. siHPRT-containing LNPs formulated in PBS, histidine, and Tris were compared to assess buffer impact on B MC3 1 integrity and C byproduct 2 formation. D The electrophilic nature of 2 is observed to react with nucleic acid cargoes, generating RNA-lipid adducts. Additional testing quantified the percentages of E RNA-lipid adducts and F intact siHPRT antisense strand. Source data are provided as a Source Data file.
We anticipated that the oxidation of MC3 1 into E,Z-conjugated dienone 2 could be deleterious for siRNA-LNP stability in at least three ways. First, an alkene conformational change (from Z,Z to E,Z) could impact drug product potency, as previously highlighted9, and influence lipid packing. The latter hypothesis was corroborated by correlating LNP particle size versus the amount of dienone 2 (Supplementary Fig. 14), though a more detailed investigation would be needed to understand if there is truly a causative effect. Secondly, the introduction of a hydrogen bond acceptor into the lipid tail—namely the ketone—could affect RNA-lipid interactions. The importance of hydrogen bonding interactions in ionizable lipid chemistry has previously been highlighted35,36. Molecular dynamics (MD) simulations and subsequent calculation of radial distribution functions between siHPRT and dienone 2 demonstrated that hydrogen-bonding between the ketone in the lipid tail and RNA is one-sixth that of the interaction between 2’s tertiary amine head group and RNA (Supplementary Fig. 15A, B). This data suggests oxidative degradants may introduce new non-covalent interactions between ionizable lipids and the RNA cargo. It is worth noting that the siRNA sequence used here is entirely methylated at the 2’-OH position, but that additional MD simulations with unmodified RNA sequences demonstrated hydrogen-bonding between the RNA’s native 2’OH position and the ketone of 2 (Supplementary Fig. 15C).
Finally, we hypothesized that the dienone motif within 2 could act as a strong electrophile, making the lipid byproduct prone to nucleophilic attack (Fig. 4A). We formulated a single batch of siHPRT-LNPs that was then buffer exchanged via desalting columns into three different formulations: (i) PBS (1X), (ii) Histidine (10 mM), and (iii) Tris (10 mM). LNPs were then stored at elevated temperatures of either 25 or 40 °C and analyzed over four weeks. The amount of intact MC3 1 was reduced to ~65–70% for LNPs in both the phosphate and Tris-containing buffers, while LNPs in histidine maintained >97% intact MC3 (Fig. 4B). LC/MS analysis revealed the primary degradant byproduct was E,Z-dienone 2, which was produced at a rate of ~8% over 28 days in the phosphate and Tris buffers but mitigated to <1% in the histidine buffer (Fig. 4C). A full table of MC3 and its related byproducts is supplied in Supplementary Table 6. Interestingly, LC/MS analysis of the siHPRT cargo revealed that both the sense and antisense strands were significantly lipidated—after four weeks at 40 °C, about 13% of the antisense strand was lipidated after encapsulation in LNPs within phosphate buffer, and about 68% of the antisense strand was lipidated in LNPs within Tris buffer (Fig. 4E). By comparison, only ~0.1% of lipidated antisense strand was detected for LNPs in histidine buffer (Fig. 4E). In addition, unwanted PS-PO conversion was noted, occurring one, two or even three times per strand, decaying the amount of intact antisense strand to 12% or 1% when LNPs were stored in phosphate or Tris-containing buffers, respectively (Fig. 4F). Histidine buffers retained 85% intact antisense strand over the same stressed conditions, a marked improvement (Fig. 4F). A full table of siHPRT sense, antisense strands and related byproducts, including siRNA-lipid adduct formation, is supplied in Supplementary Table 7. While lipid oxidation, dienone 2 generation, and siRNA lipidation are all correlated (Fig. 4A–C), we sought further evidence that dienone 2 was responsible for reactivity with encapsulated cargo. It is known that Michael acceptors can react with positions in nucleobases37,38, as well as phosphorothioate linkages in the backbone39. Tandem MS on MC3-modified sense and antisense strands first revealed a distribution of molecular weights for the two strands that differed by ~15.98 daltons, indicative of the PS-PO conversions discussed previously (Supplementary Fig. 16A, B). More strikingly, MS analysis of lipidated siRNA byproduct revealed molecular weight distributions that were larger than the parent molecular weights by 655.57 daltons—a value within instrumental error for E,Z-dienone 2’s exact mass (655.59 ± 0.06 daltons) (Supplementary Fig. 16A, B).
Lipidation of siRNA occurred up to two times per strand, albeit less commonly (e.g., 13.2% and 2.4% of antisense strand was lipidated once or twice, respectively, after 6 months of storage in PBS 1X (Supplementary Fig. 16, Supplementary Table 2)). Although the exact site of lipidation is still under investigation, we believe that modification through nucleobase addition is most likely, as (i) addition via phosphorothioate would result in a 1 dalton-larger molecular weight shift, and (ii) one would expect that the siRNA strand’s molecular weight distributions associated with PS-PO conversion would be less likely to form lipid adducts, which was not observed (Supplementary Fig. 16A, B). Collectively, these data support a mechanism of unintentional RNA lipidation: oxidation of the unsaturated lipid tails within MC3 1 results in the production of electrophilic E,Z-dienone byproduct 2, which then reacts with neighboring RNA to form RNA-lipid adducts (Fig. 4D). DFT calculations were performed to understand the formation of RNA-lipid adducts using a model base guanine, with a lipid model system (3E,5Z)-hepta-3,5-dien-2-one. The activation enthalpies were determined as 20.0 and 24.7 kcal/mol for 1,6 and 1,4-addition, respectively, indicating that 1,6 addition is favorable.
This mechanism is conceptually similar to that reported recently2, with a number of key distinctions: in the cited work (i) the ionizable lipids were primarily saturated, (ii) the oxidative byproducts highlighted were produced through head group N-oxidation, (iii) the problematic electrophilic degradants were aldehydes, (iv) lipid adducts were formed with mRNA, a single-stranded nucleic acid with solvent-exposed nucleobases, and (v) formulation strategies to mitigate this degradation were not proposed. With knowledge of the proposed mechanism and the implications of shutting down lipid oxidation, we envisioned that many other excipients could be used to improve drug product stability. We formulated a large batch of siHPRT-LNPs and buffer-exchanged aliquots through small-scale desalting columns into a number of formulations (Table 1), with excipients including EDTA, imidazole, methionine, sodium ascorbate, tryptophan, NAc-tryptophan, acetate, citrate, and Tris. After an abbreviated study of four weeks at room temperature, the critical quality attributes of each siHPRT-LNP solution were measured and summarized in Table 1. Aside from histidine, the excipients that promoted stability best included methionine, tryptophan, EDTA, and citrate. We believe that the latter two excipients impart particle stability by chelating trace metals that are otherwise capable of catalyzing lipid oxidation, as forced degradation experiments on MC3 1 in solution (not formulated in an LNP) demonstrated susceptibility to oxidation in solutions containing 1 ppm nickel, iron and copper (Supplementary Fig. 17A, C).
Optimized siRNA-LNP formulations retain potency after extended storage at room temperature
Finally, we tested the knockdown efficiency of siHPRT-LNPs within HeLa cells after LNPs were stored for one month at either 2–8 °C or at room temperature. A head-to-head comparison was again done between two buffers, 1X PBS (pH 7.4) and 10 mM histidine and 140 mM NaCl (pH 6.0). siHPRT samples incubated with or without the transfection agent RNAiMax were used as positive and negative controls, respectively. On the day of transfection, fresh LNPs were generated, buffer exchanged into 1X PBS, and compared to aged LNP samples. After a 24-hour transfection, RNA was isolated via a Cells-to-CT assay kit and HPRT gene expression was quantified by qPCR (Fig. 5A). We observed that LNPs stored at refrigerated temperatures in either PBS or histidine-containing buffers had similar knockdown efficiencies to that of fresh LNPs. However, LNPs stored in PBS at room temperature lost potency, with no detectable IC50 at these concentrations. On the other hand, LNPs stored in histidine buffer at room temperature for one month (red line) remarkably retained potency, with IC50 values similar to those of fresh or refrigerated LNPs (Fig. 5B). For example, at a concentration of 0.1 nM siRNA, HPRT expression is effectively knocked down to 42 ± 2% by fresh LNPs, as well as LNPs refrigerated in PBS (ns, p-value of 0.65), and LNPs refrigerated or at room temperature in histidine buffer (ns, p-values of 0.85 and 0.79). Conversely, HPRT expression after incubation with LNPs at room temperature in PBS has negligible knockdown, with a relative HPRT expression of 96% (p-value of 0.02 when compared to fresh LNPs). These data support that siRNA-LNPs stored in histidine-containing buffers retain their biological function even at elevated temperatures, while those stored in phosphate-containing buffers undergo a temperature-dependent loss in potency.
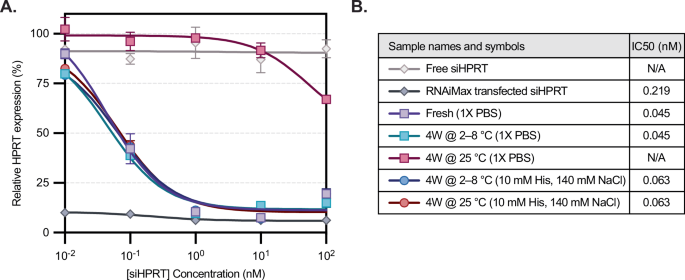
A Relative mRNA expression was measured in response to increasing sample concentration (n = 2 independent replicates), normalized to an untreated control. Except for fresh LNP formulated on the day of assay, all LNP samples were stored at 2-8 °C or 25 °C for four weeks in 1X PBS or 10 mM histidine buffer. siHPRT incubated with RNAiMax and free siHPRT were used as positive and negative controls, respectively. Markers and error bars represent the mean and standard deviation between experimental duplicates. At 0.1 nM siRNA, HPRT expression was compared across groups to fresh LNPs using a two-tailed Student’s t-test assuming unequal sample variance. LNPs stored in PBS at refrigerated temperature, or LNPs stored in histidine buffer at either refrigerated or room temperature, showed no significant difference (p-values > 0.05), while LNPs at room temperature in PBS resulted in negligible knockdown (p = 0.02). B IC50 values for the samples were determined using nonlinear regression. N/A refers to samples for which a plateau was not established over the tested concentrations. Source data are provided as a Source Data file.
In summary, we have shown that degrees of unsaturation in ionizable lipids are prone to degradation through an oxidative pathway that results in electrophilic degradants. These problematic impurities can react with neighboring RNA cargoes, forming RNA-lipid adducts. Degradation is observed at high levels (>40 mol%) for LNPs formulated in common phosphate-based matrixes. We show that this degradation can be mitigated by optimizing the drug product matrix; for example, through the use of mildly acidic, antioxidant-containing histidine formulations. Histidine-based formulations are shown to improve siRNA-LNP stability, ultimately extending the room temperature shelf-life from two weeks to upwards of six months to date. Finally, it is shown that the bioactivity of model siRNA-LNPs can be retained for at least a month at room temperature, illustrating the significance of ensuring adequate colloidal, lipid, and siRNA drug substance stability in siRNA-LNP systems.
This work highlights challenges that academic and industrial researchers must be cognizant of when developing these multicomponent drug products, and the ability of drug product matrix optimization to solve them. However, a limitation of the work described herein is that it primarily focuses on siRNA-LNPs, which are comparatively more stable than mRNA-LNP systems, whose shelf-life is limited by the fragility of the mRNA cargo. While preliminary work demonstrates that the described formulation solutions can be applied to more sensitive cargoes, including mRNA, the benefits are dependent on ionizable lipid chemistry and are most pronounced with unsaturated lipids (Supplementary Fig. 18, Supplementary Table 8). As such, more comprehensive, long-term stability studies using these systems are necessary. Advancements in this field are expected to directly impact the shelf-life of these complex materials, convenience in their administration (e.g., improving in-use stability profile), or reduce the need for distribution of RNA-LNP drug products within a frozen cold chain to expand patient accessibility.