Immunotherapy is rapidly redefining the treatment landscape for gastric cancer—not only in metastatic settings, but now reaching into the neoadjuvant, perioperative, and even organ-preserving space. Across Asia, Europe, and the U.S., trials are testing PD-1 inhibitors alone or in combination with chemotherapy, anti-angiogenic agents, and novel strategies like oncolytic viruses and TIGIT blockade.
From deep immune profiling of tumor microenvironments to real-world studies of frail populations, the goal is no longer just palliation—but personalization, precision, and potential cure. Whether in MSI-H resectable tumors or liver-limited metastatic disease, checkpoint blockade is being pushed earlier, deeper, and more selectively into the gastric cancer continuum.
Tumor Microenvironment Study in Gastric Cancer
This ongoing observational cohort study at the Chinese PLA General Hospital (Beijing) is exploring how the tumor microenvironment (TME) changes in advanced gastric cancer patients treated with S-1 + oxaliplatin (SOX) chemotherapy combined with a PD-1 inhibitor (nivolumab or sintilimab).
Patients undergo gastroscopic biopsies before treatment and after 2–8 cycles of therapy. Outcomes are compared across responders (CR/PR) and non-responders (SD/PD) to identify immune and molecular factors that influence efficacy.
- Design: Single-center, observational (n=28, planned)
- Population: Advanced gastric adenocarcinoma, ECOG 0–1, ages 18–70
- Intervention: SOX + PD-1 inhibitor; biopsies pre- and post-treatment
- Primary endpoint: Single-cell transcriptomic and immune repertoire profiling
- Timeline: Started July 2022; primary completion expected June 2024
This trial leverages single-cell sequencing to clarify how immunochemotherapy reshapes the TME and to uncover predictors of treatment response.
Real-world Camrelizumab ± Apatinib/Chemotherapy in Advanced Gastric Cancer
This large prospective observational study (n≈504) led by Changzhi People’s Hospital, China, is assessing the safety and effectiveness of camrelizumab given alone or combined with apatinib and/or chemotherapy in routine practice for advanced gastric cancer.
- Design: Real-world, multicohort, prospective
- Cohorts: First-line: camrelizumab ± apatinib/chemo; Second-line: camrelizumab ± apatinib/chemo; Third-line+: camrelizumab ± apatinib/chemo.
- Population: Adults 18–75, advanced gastric cancer (RECIST 1.1 measurable), no prior immunotherapy or anti-angiogenic therapy
- Interventions: Camrelizumab 200 mg IV (q2–3w); apatinib 250 mg daily; investigator’s choice chemo
Primary endpoint: Objective response rate (ORR, 24 months) - Key secondary endpoints: PFS, DCR, OS (all up to 24 months)
- Timeline: Started Nov 2023; primary completion Oct 2025; final completion Oct 2026
The trial aims to reflect real-world outcomes and subgroup responses while also exploring potential prognostic biomarkers for camrelizumab-based therapy in gastric cancer.
APICAL-GC: Anlotinib + Toripalimab as First-line Therapy in Advanced Gastric Cancer
The APICAL-GC study is a phase II, open-label, single-center trial being conducted at Shanghai Changzheng Hospital to evaluate the efficacy and safety of combining anlotinib with toripalimab as first-line treatment for advanced gastric cancer in patients with an ECOG performance status of 2. Anlotinib is an oral multitargeted tyrosine kinase inhibitor that blocks VEGFR, FGFR, PDGFR, and c-kit, while toripalimab is a PD-1 inhibitor designed to restore antitumor immune activity. Together, the combination aims to provide a tolerable and effective therapeutic option for frail patients who are often excluded from standard clinical trials.
Eligible participants are adults with histologically confirmed stage IV gastric cancer, no prior systemic treatment, at least one measurable lesion, and adequate organ function. Patients with HER2-positive tumors or dMMR/MSI-H disease are excluded. Treatment consists of anlotinib 12 mg orally on days 1–14 of a 21-day cycle, combined with toripalimab 240 mg intravenously on day 1 of each cycle.
The primary endpoint is objective response rate according to RECIST 1.1. Secondary outcomes include progression-free survival, overall survival, depth of response, disease control rate, and safety. The study plans to enroll approximately 24 patients. It began in February 2020, with primary completion expected in October 2024 and final completion in December 2024.
By focusing on a vulnerable ECOG 2 population, this trial seeks to determine whether the dual approach of VEGFR inhibition and PD-1 blockade can achieve meaningful responses while maintaining manageable toxicity in advanced gastric cancer.
Adjuvant Chemotherapy ± Immunotherapy in High-Risk Esophageal and EGJ Cancer
This randomized controlled trial from The Second Hospital of Shandong University is evaluating whether adding immunotherapy to standard adjuvant chemotherapy ± radiotherapy can improve outcomes for patients with resected esophageal or esophagogastric junction (EGJ) cancer who are at high risk of recurrence (R1 resection and/or nodal involvement after surgery, with or without prior neoadjuvant therapy).
- Design: Phase not specified; randomized, parallel assignment, double-masked (investigator and assessor)
- Population: 200 patients, ages 25–80, with resectable esophageal or EGJ cancer (Siewert I–II), excluding Siewert III or metastatic disease
- Arms: Control group receives chemotherapy ± radiotherapy as standard adjuvant treatment, while the experimental group receives chemotherapy combined with immunotherapy ± radiotherapy.
- Primary endpoint: 5-year disease-free survival (DFS)
- Secondary endpoints: Overall survival (OS), safety/toxicity (CTCAE v5.0), and quality of life (KPS, PS, QOL, EORTC QLQ-C30)
- Timeline: Began July 2020; primary completion expected Dec 2023, final completion by Dec 2027
This trial directly addresses the unmet need in patients with residual disease or nodal positivity after surgery, aiming to determine whether immunotherapy can improve long-term survival beyond chemotherapy alone.
ZODIAC: Zimberelimab ± Domvanalimab in Resectable MSI-H/dMMR Gastric and GOJ Adenocarcinoma
The ZODIAC trial is a UK multicenter, randomized phase II study led by the Royal Marsden NHS Foundation Trust, testing a chemotherapy-sparing perioperative immunotherapy strategy in resectable mismatch repair deficient (MMRd) / MSI-H gastric and gastro-oesophageal junction adenocarcinoma.
Patients are randomized 1:1 to receive either zimberelimab (anti–PD-1) monotherapy or zimberelimab combined with domvanalimab (anti-TIGIT) every 3 weeks before surgery, with the option to continue postoperatively.
- Primary endpoint: Pathological complete response (pCR) rate at surgery
- Secondary endpoints: Clinical complete response, R0 resection rate, radiological response, event-free survival, overall survival, safety/tolerability, and surgical outcomes
- Exploratory analyses: Translational biomarker studies (ctDNA, TMB, PD-L1, TCR sequencing, RNA/WES) to identify predictors of response or resistance
The study opened in February 2025, aims to enroll 50 patients, with primary completion expected in March 2027 and final follow-up in 2031.
By focusing on MMRd/MSI-H tumors, which are highly immunogenic, ZODIAC seeks to determine whether immune checkpoint inhibition alone, or in combination with TIGIT blockade, can replace chemotherapy in the perioperative setting.
First-Line and Neoadjuvant Immunotherapy for Gastric Cancer
This is a prospective observational cohort study from Qilu Hospital of Shandong University designed to evaluate the real-world efficacy, safety, and biomarker profiles of immune checkpoint inhibitors (ICIs) combined with chemotherapy in patients with locally advanced or advanced gastric cancer. Patients either receive ICI plus chemotherapy or chemotherapy alone, depending on clinical decision-making.
The study plans to enroll 500 patients who have not received prior anti-cancer treatment. Blood, stool, and tumor tissue samples are collected for biomarker analysis.
- Primary endpoint: Overall survival (OS)
- Secondary endpoints: Disease control rate, progression-free survival, objective response rate, duration of response, disease-free survival, major pathological response, and treatment-related adverse events (CTCAE v5.0).
Recruitment began in January 2022, with primary completion expected in January 2027 and final completion in 2028.
By integrating clinical outcomes with biomarker studies, this trial aims to clarify the role of immunotherapy as first-line and neoadjuvant therapy in gastric cancer and guide patient selection for future treatment strategies.
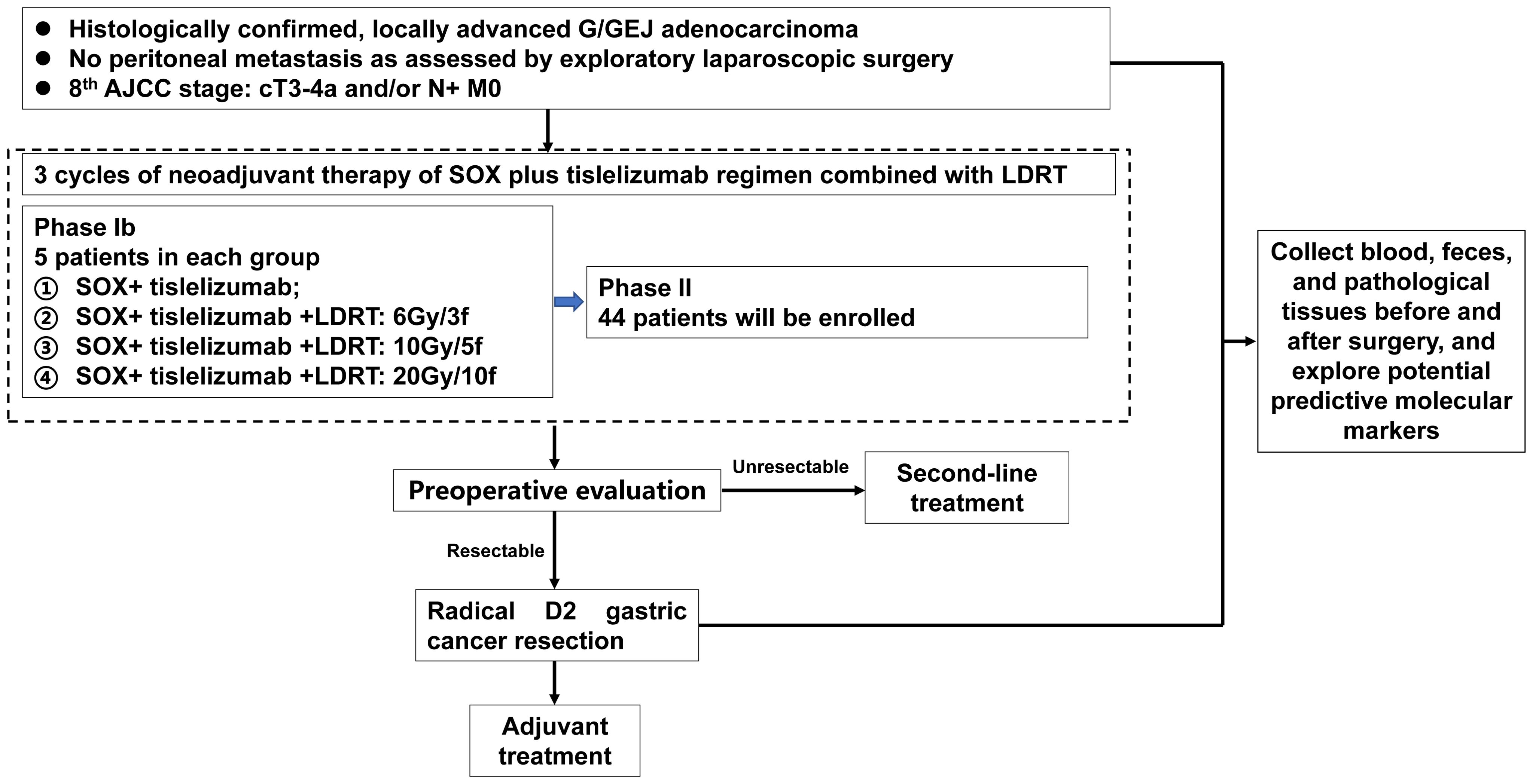
Tislelizumab Plus SOX in Gastric Cancer With Liver Metastases
This exploratory phase II/III study from Zhejiang University is testing Tislelizumab, a PD-1 inhibitor, combined with SOX chemotherapy (S-1/Tegafur + Oxaliplatin) in patients with gastric or gastroesophageal junction adenocarcinoma limited to the liver. Eligible patients must have up to three measurable liver metastases (≤5 cm each), no prior systemic therapy, and ECOG 0–1.
The regimen involves six cycles of Tislelizumab plus SOX every 21 days. The primary endpoint is objective response rate (ORR) after six cycles, with progression-free survival (PFS) as a key secondary endpoint.
The trial, which began in April 2022 and expects completion by December 2025, aims to determine whether adding PD-1 blockade to chemotherapy can overcome the limited survival benefit of standard regimens in this high-risk subgroup.
Phase II Trial of OBP-301 (Suratadenoturev) Plus Pembrolizumab in Immunotherapy-Refractory Esophagogastric Adenocarcinoma
This phase II study at Weill Cornell Medicine is evaluating suratadenoturev (OBP-301, telomelysin), an oncolytic adenovirus, in combination with pembrolizumab for patients with advanced or metastatic gastric, gastroesophageal junction (GEJ), or esophageal adenocarcinoma who have progressed after prior PD-1 therapy.
Patients receive intratumoral OBP-301 injections every two weeks (4 planned, 1 optional) via endoscopic guidance, paired with pembrolizumab infusions every six weeks for up to two years. The goal is to test whether OBP-301 can overcome immunotherapy resistance by promoting tumor lysis and immune activation.
The primary endpoint is objective response rate (ORR), with a target of 20% compared to the <5% expected from PD-1 continuation alone. Secondary measures include duration of response, progression-free survival, overall survival, and safety. The trial uses a Simon two-stage minimax design: 13 patients in stage one, with expansion to 27 if at least one response is seen.
Immune Checkpoint Inhibitors for Organ Preservation in Non-metastatic dMMR/MSI-H Gastric Cancer
This phase II study (NCT06580574), led by Peking University, is investigating immune checkpoint inhibitors for organ preservation in non-metastatic dMMR/MSI-H gastric and colon cancers. Patients first receive PD-1/PD-L1 antibody monotherapy for up to 24 weeks. If a (near) complete clinical response is not achieved, treatment escalates to an intensive regimen of sintilimab, IBI310 (anti-CTLA4), and lenvatinib (anti-VEGF). Patients who still do not reach response milestones are offered radical surgery.
The primary endpoint is the rate of organ preservation, defined as patients achieving cCR or near-cCR without surgery. Secondary endpoints include long-term organ preservation rates, response and disease control with the combination regimen, survival outcomes (DFS, OS, recurrence-free survival), perioperative safety, and quality of life. Exploratory analyses will assess immune cell subsets, tumor gene alterations, and imaging correlates linked to response.
The trial aims to enroll 38 patients, with completion expected in 2029, to test whether immunotherapy-based approaches can safely avoid surgery in selected patients with localized dMMR/MSI-H gastric or colon cancer.
Predictive Model for Gastric Cancer Immunotherapy Response
This prospective, observational study at Changzhi People’s Hospital is exploring how tumor-infiltrating lymphocytes (TILs) and the histone modification H3K4me3 influence response to immunotherapy in advanced gastric or gastroesophageal junction adenocarcinoma.
- Rationale: Immunotherapy has transformed gastric cancer treatment but benefits remain inconsistent. Changes in the tumor microenvironment (TME), particularly in TIL density/distribution and epigenetic regulation via H3K4me3, may predict treatment efficacy.
- Design: Case–control registry; ~170 patients, ages 18–75, with locally advanced unresectable or metastatic disease scheduled for first-line ICI plus chemotherapy (± anti-HER2).
- Assessments: Multiplex immunofluorescence, Co-IP, immunohistochemistry, and in situ hybridization to profile TILs, H3K4me3, PD-L1, HER2, MMR, KMT/KDM enzymes, and EBER; plus blood biomarkers (cytokines, NLR, PLR, SII, tumor markers).
- Groups: Patients will be classified as high-response (CR/PR) or non-response (SD/PD) after immunotherapy.
- Endpoints: Primary outcome is immunotherapy response prediction over 1 year.
- Timeline: Began Jan 2025; primary completion Jan 2026; final completion Dec 2026.
The goal is to build a predictive model integrating TILs and H3K4me3 signatures to better identify patients most likely to benefit from immunotherapy, supporting personalized gastric cancer treatment.

You Can Also Read About 10 Ongoing Clinical Trials on Immunotherapy in Lung Cancer
Written by Toma Oganezova, MD, Editor-in-Chief of OncoDaily IO
